Return to:
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
CORROSION INHIBITION
Norman Hackerman
President Emeritus, Rice University
President Emeritus, The University of Texas at Austin
Department of Chemistry and Biochemistry
Austin, TX 78712, USA
(August, 2006)
Corrosion reactions involve a metal or metal alloy and its fluid surroundings. All metals and alloys are susceptible to corrosion by one or more naturally available or man-made fluids.
These reactions may cause a uniform loss of metal with consequent loss of cohesive strength or simply an unacceptable change in appearance, for example tarnishing of gold or silver. There can be local losses involving much less metal but leading to diminished whole piece strength. Localized corrosion is exemplified by pitting or stress corrosion cracking.
Control of the corrosion rate can be affected by reducing the tendency of the metal to oxidize, by reducing the aggressiveness of the medium, or by isolating the metal from the fluid. The latter can be done by coating the metal with a millimeter thick impervious non-corroding coating. These have wide spread use, but their effect may not be permanent because of breaks in the coating over time. Also in some systems coating might interfere with the process for which the equipment is used because they might change the heat transfer properties for example.
In cases where a fairly thick coating is not acceptable, the use of corrosion inhibitors comes into play. These chemicals are continually fed into the fluid with the objective of having them move to the metal-fluid interface. There the intact inhibitor molecule attaches to the metal, or it reacts with the surface to form a thin adherent compound. In the first case they act by adsorption (Toth, 2002). In either case, the films are only one to a few molecules thick, that is nanometer thickness.
Some inorganic (non-carbon) chemicals like the chromates function in both ways and do so very well. However they are hazardous to human and animal health and are not normally used now. Other inorganics like phosphates, borates, nitrites, and silicates function by reaction to form micrometer thick films. They are used for some metals in near neutral (pH ~ 7) aqueous solutions, for instance, in water treatment plants.
For many aqueous systems, for elevated temperature equipment, for crude oil production, etc. organic (carbon based) chemicals find more use. These materials are likely to function by adsorption. Here the organic molecule, which orients itself suitably, becomes attached to the solid surface often via a less than total reaction between the inhibitor molecule and the solid surface. The attachment does not require a total electron transfer in either direction. A coulumbic force, for example ion-dipole attraction, suffices to attach the inhibitor molecule to the solid surface. This in turn interferes with access of the corrosive entity to the surface.
The adsorbed layer can be formed all over the surface either in a single layer or as a multilayer or a mixture of both. The more complete the coverage the better. This process has the advantage of being molecularly thin and thus not too intrusive in heat conduction for example.
But there are problems. The amount of a given material adsorbed from a mixture depends on its concentration, temperature, fluid flow rate, as well as on the nature of the adsorbent that is, the solid surface. The film has to be kept intact by continually adding inhibitor to the medium to maintain a predetermined concentration of inhibitor. The continuing concentration is generally lower than that used initially, but both are at the milli-molar level.
That is not all. If temperature or flow rate of the system changes, the amount adsorbed is apt to change. For temperature, the change is energetic, a basic change in the amount adsorbed. For fluid flow, the change is dynamic, that is a change of fluid movement at the interface. This may affect the amount left at the interface. Faster flow generally causes removal of some of the physically adsorbed material from the solid surface.
To this point the object was to get you to visualize the system. The point now is to get some insight into how the efficiency of inhibition is determined. Corrosion rates can be measured in a variety of ways. In fact, corrosion rates can be determined from any measurable change available. Examples are metal weight loss, rate of gaseous production, and changes in solution composition. The basic approach is to expose small pieces of the metal in question to the fluid environment, preferably under flow conditions. Measurement of the corrosion rate can be carried out electrochemically or chemically. It is best to acquire not only the initial rate but also the steady rate. The latter is more useful for practical purposes.
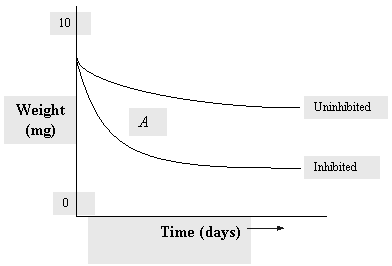 |
| Fig. 1. Weight loss of coupons. |
For weight loss, plot the change in weight of each cleaned coupon (metal sample) both before and after exposure using multiple samples. Each one of these can be removed at predetermined times, see the representative curve Figure 1. The slope of the curve at any point is the corrosion rate at that time. Note that the rate past point A is not quite flat. The bottom curve shows the corrosion rate for the inhibited system. The weight loss of the inhibited coupon divided by the loss of the uninhibited coupon provides a measure of inhibition efficiency.
To repeat, the rates can be determined from any quantity which changes regularly as a function of time. Thus, change in solution concentration of iron (for steels), pH of the solution, or weight of metal coupon can be used.
There are some caveats. Laboratory experiments are useful in screening candidate inhibitors. Following that, they should be subjected to a lab system that emulates conditions in the field, composition of the fluid, temperature and its changes, flow rates and their changes, time, and so on. Then there should be tests in the real system itself, at least long enough to cover any condition cycles the system may have. However, continuous exposure with ongoing data output is best.
To repeat, inhibitors are useless if they do not reach the metal surface intact. They can be lost by reaction with chemicals in the stream or to �thieves� in the system, that is any other solid surface exposed to the fluid stream like sand. Also, the inhibitor should not be detrimental to either process or product. Further, since the system may change with time, so must the corrosion control. Thus the control system must be monitored consistently.
The question may be asked, why go to all of the trouble that is entailed in using corrosion inhibitors? There are two important reasons to do so.
First, there is the matter of safety. Industrial corrosion can lead to process breakdowns that culminate in explosions or the venting of chemicals dangerous to health. In either case the ounce of prevention looms large over the pound of cure.
Second, there is the matter of cost. An article in 1995 (Battelle) estimated the cost of corrosion in the United States per year was $300 billion, an appreciable portion of the Gross Domestic Product. It also estimated that 30% of that amount could be saved by corrosion control, which of course includes corrosion inhibition.
Related articles
Anodizing
Cathodic/anodic corrosion protection
Electrochemistry of corrosion
Bibliography
- Fundamental Behavior of Model Corrosion Inhibitors, M. Knag, "Journal of Dispersion Science and Technology" Vol. 27, pp 587-597, 2005.
- Adsorption: Theory, Modeling, and Analysis, J. Toth (editor), Marcel Dekker, New York, 2002.
- Corrosion Inhibitors: Principles and Applications, V. Sastri, Wiley New York, 1998.
- Economic Effects of Metallic Corrosion in the United States, Battelle Columbus Laboratories, Columbus OH, 1995.
- Corrosion Inhibitors: an Industrial Guide (2nd edition), E. Flick, Noyes, Park Ridge NJ, 1993.
- Chemical Inhibitors for Corrosion Control, B. G. Clubley (editor), Royal Society of Chemistry, Cambridge, UK 1990.
- Organic-Compounds as Corrosion-Inhibitors in Different Environments - a Review, B. Sanyal, "Progress in Organic Coatings" Vol. 9, pp 165-236, 1981.
Listings of electrochemistry books, review chapters, proceedings volumes, and full text of some historical publications are also available in the Electrochemistry Science and Technology Information Resource (ESTIR). (http://knowledge.electrochem.org/estir/)
Return to:
Top –
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
|