Jaroslav was born on the 20th of December 1890, into the family of Leopold Heyrovsky, professor of Roman law at Charles University in Prague, then in the Austro-Hungarian Empire. He had three elder sisters and one younger brother. The two boys showed keen interest in nature from their childhood, the younger one later became one of the leading Czech entomologists, while the elder one's preferred subjects in school were biology, mathematics, and physics (chemistry was not taught at that time). When William Ramsay was distinguished by the Nobel Prize in Chemistry in 1904 for his discovery of rare gas elements, Jaroslav, strongly impressed, decided that in the future he would like to study physical chemistry. After the first year of Jaroslav's study at Prague University, as physical chemistry was not an independent subject there, father Leopold agreed to support his son's continued studies at the University College in London (Figure 2), where William Ramsay held the chair of general chemistry. There the Czech student had the privilege to attend the great chemist's lectures. In 1913 Ramsay retired, succeeded by the electrochemist F. G. Donnan, who affected decisively the scientific orientation of young Heyrovsky by suggesting to him as topic for Ph.D. thesis the electrode potential of aluminum. For the experimental measurement, in order to avoid formation of passivation layers on the aluminum surface, Donnan advised his student to use dilute aluminum amalgam slowly flowing out of a capillary tube thus constantly renewing its surface. This acquainted Heyrovsky with the sphere of problems which he was later facing in his work with the dropping mercury electrode.
Back home for summer holidays in 1914, caught by the outbreak of World War I, Heyrovsky was called up to join the Austrian army and to serve in military hospitals as dispensing chemist and x-ray technician. This position gave him the chance, in rare moments of leisure, to work on his dissertation "On the electroaffinity of aluminum", and so in last days of the war, in the autumn of 1918, he could present himself, in military uniform and with a completed dissertation, for a Ph.D. examination at Prague University. His examiner in physics on that occasion was Professor Bohumil Kucera, who asked the candidate about the method of measuring surface tension of polarized mercury by dropping mercury electrode. Heyrovsky, knowing Kucera�s work, was prepared for such question, and the surprised examiner suggested that the student continue, after successful examination, his own not fully accomplished research – to solve the so-called "Kucera's anomaly" of electrocapillary curves obtained by the dropping mercury method.
After the war, as a fresh Ph.D., Heyrovsky became assistant at the Department of Inorganic and Analytical Chemistry at the Prague University, this time in the new Czechoslovak Republic. Following Kucera's instructions, he started weighing drops of mercury collected in various solutions from the dropping mercury electrode under different values of voltage applied to it with the negative pole of a voltage source, while the layer of mercury at the bottom of the cell was connected to the positive pole. The measurements were very tedious: for each value of applied voltage he had to count 100 drops falling into a small spoon submerged in the solution, to dry the collected mercury, to weigh it exactly, to record the weight, to change the voltage by 10 mV, and to continue, over the entire voltage range of 2 to 3 volts. A certain simplification was to measure drop-time, proportional to the surface tension, under the assumption of constant rate of flow of mercury at various potentials. The drop-weight-voltage, or drop-time-voltage curves had mostly the correct shape of the electrocapillary parabola, but sometimes their round maximum was overlapped by a secondary sharp maximum – the Kucera's anomaly. In some cases, Heyrovsky observed that the drop-weight, or the drop-time, remained constant over a certain voltage range; it occurred to him that this might indicate passage of electrolytic current, and he decided from then on to measure current, passing through the cell, in addition to the drop-weight or drop-time.
|
 |
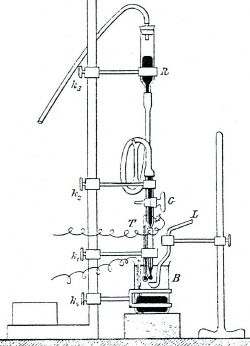
| Fig. 3. Apparatus for measuring current-voltage curves in electrolysis with dropping mercury electrode. |
In the afternoon of Friday, the 10th of February 1922, he connected a sensitive mirror galvanometer, provided with a lamp irradiating the mirror, to the electrolytic circuit with the dropping mercury electrode dipping into 1 M solution of sodium chloride (common salt), and followed motions of the luminous spot, reflected from the mirror of the galvanometer, on the measuring scale (Figure 3). The instantaneous current oscillated rhythmically according to the regular growing and falling of the drops. After the mean values of the current oscillations were plotted as a function of the voltage applied to the electrodes, the resulting polarization curve showed two equally high steps of current increase, about 0.8 V apart, and a final steep increase of current out of scale, corresponding to the highest value of 2.0 applied volts. In contrast with the hitherto use of solid electrodes, this curve turned out to be exactly reproducible. It was the first polarization curve obtained with the dropping mercury electrode, this particular one visualized the two-step electrolytic reduction of molecular oxygen dissolved from the air under atmospheric pressure in 1 M solution of common salt. The height of the current steps was proportional to oxygen concentration in the solution, and the position of the steps on the voltage scale appeared to be values characteristic of oxygen. Thanks to the minute size of the mercury drops the amount of oxygen consumed in the electrolytic reaction was completely negligible. Usefulness for analytical chemistry of the electrolysis with dropping mercury electrode appeared straightforward. That day can be considered as the date of birth of the method, which later received the name "polarography".
 |
| Fig. 4. Masuzo Shikata and Jaroslav Heyrovsky on the occasion of Faraday Discussion in London (December, 1923). |
After the successful initial experiment, Heyrovsky continued a systematic research of electrolysis, first with the dropping mercury cathode, as at the beginning various reduction processes were studied, for which mercury, with its highest hydrogen overpotential of all metals, offers the widest attainable range of potentials in the negative direction. Later it was shown that even oxidation processes can be followed, although limited by anodic dissolution of metallic mercury; and in a relatively narrow positive potential range, the dropping mercury electrode then can serve also as an anode. In April of 1922, Heyrovsky was appointed assistant professor in the newly established department of physical chemistry, and students as well as interested colleagues were readily joining him in his original research. His first account of "Electrolysis with the dropping mercury cathode" was published in Czech in 1922, an English version appeared in the "Philosophical Magazine" in 1923. The latter article attracted the attention of a young Japanese scientist, Dr. Masuzo Shikata, to the extent that he came to Prague in 1923 to learn the new method on the spot. In November of the same year, Heyrovsky and Shikata attended the "General Discussion on Electrode Reactions and Equilibria" of the Faraday Society in London, to which each contributed with communication on electrolysis with dropping mercury electrode (Figure 4).
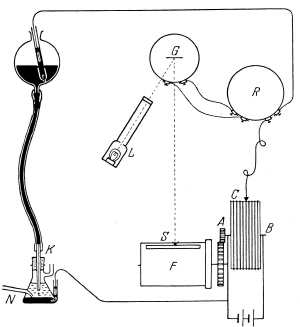 |
| Fig. 5. Scheme of automatic measuring circuit with polarograph. |
The only drawback of the promising new method was the cumbersome way of manual recording of the curves. This aspect was discussed at length between Heyrovsky and Shikata, and on the 30th of March 1924, a sketch was submitted to the workshop of the physics department in Prague of an instrument for automatic photorecording of current-voltage curves (Figure 5). The result proved successful, and in a 1925 special issue of the Dutch journal "Recueil des Travaux Chimiques des Pays Bas", as part of a special series of eleven papers on "Researches with the dropping mercury cathode", a paper was published by Heyrovsky and his coworkers describing the new device of Heyrovsky and Shikata. The paper was entitled "The polarograph", meaning an instrument for recording the process of electrolytic polarization. Although it was not specified in that paper that the instrument should be used solely for measurements with the dropping mercury electrode, the method of electrolysis with that kind of electrode has gradually acquired the name "polarography". The polarograph shortened the time of recording polarization curves from hours to minutes and, moreover, it automatically provided an objective document of exactly reproducible measurements. Thus, polarography brought the fulfillment of the Nernst's idea of an electrochemical analogy to spectroscopy (Figure 6). Moreover, polarographic analysis allowed determination of electroactive species in 10 µM concentration even in a minute volume of solution. This considerably spurred the further development and the spreading of polarography. In 1925 Shikata left Prague for home, and in his luggage he carried one of the first polarographs, which became the cornerstone of the Japanese polarographic school. Among other foreign scientists who visited Prague, in order to learn polarography at the early stage and to introduce it in their own countries, were Dr. Giovanni Semerano from Italy, then author of the very first textbook on polarography, and Dr. Wiktor Kemula from Poland.
 The first decade
The first decade
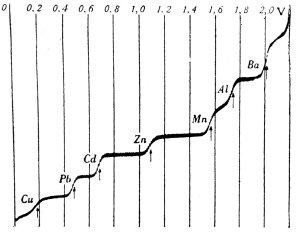 |
| Fig. 6. "Polarographic spectrum" – curve of polarographic reduction of seven different cations. |
In 1926 Heyrovsky became full professor, and polarography was going to be the primary subject of research in his department of physical chemistry. At that time, Heyrovsky and his friend, x-ray spectroscopist Dr. Vaclav Dolejsek, were preoccupied with the search for the new chemical element No 75; it appeared that polarographic curves of certain manganese ores provided results which could be ascribed to reduction of one of the oxidation states of this new element. However, Heyrovsky has proven with further polarographic experiments that, contrary to his previous belief, the manganese ores did not contain element No. 75, and he conceded the discovery of the new element to W. and I. Noddack, who called it "rhenium".
The problem of Kucera's anomaly was not forgotten: with Kucera�s assistant, Dr. Rudolf Simunek, Heyrovsky showed that the pointed maxima on electrocapillary curves appeared with solutions with which similar maxima appeared also on polarographic curves – commonly it occurred when oxygen contained in the solution was reduced at the electrode. Later it was explained that both maxima were caused by the streaming of the solution around the mercury drops when electrolytic processes, accompanied by changes of adsorption of the electroactive species, were taking place at the electrode – the streaming was rinsing the mercury surface, seemingly increasing the surface tension on the electrocapillary curves, and it increased transport of the electroactive substance to the electrode increasing the electrolytic current on the polarographic curves. Professor Kucera, unfortunately, did not witness these results – he had passed away untimely in early 1921.
In the course of the first decade of polarography, the Prague group gathered a wealth of experimental experience providing sufficient material for the development of a basic theory underlying various types of polarographic current-voltage curves. The first impulse came from Kemula, who studied the most common type of polarographic currents where the electroactive species arrives to the electrode by diffusion. He found that, in the same solution, two polarographic capillaries provide about the same current when the rate of flow of mercury through them (in milligram/second) is equal. Further he observed that the "diffusion current" increased with the square root of mercury pressure given by the height of the level of mercury in the reservoir above the tip of the capillary. Theoretical explanation of these experimental facts was soon provided by Heyrovsky's assistant, Slovak physicist Dionyz Ilkovic, who in 1934 derived the equation of electrolytic current, controlled by the rate of diffusion of the electroactive species to surface of the dropping mercury electrode. The equation was derived under the simplifying assumption of linear diffusion, and agreed well with experimental results. Almost twenty years later, the equation was precisely recalculated for spherical diffusion by another theoretician of the Prague group, Jaroslav Koutecky. According to his results, the currents should be about 10% higher than predicted by the Ilkovic equation. That was later confirmed by rigorous measurements with exclusion of the partial transfer of exhaustion of the electroactive species around the tip of the currently used vertically positioned cylindrical capillary. The good agreement of the original Ilkovic equation with experiment was thus explained by a compensation of the experimental error and the error introduced by the theoretical simplification.
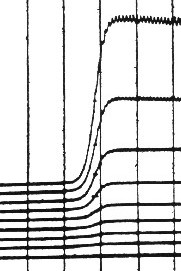 |
| Fig. 7. Polarographic curves showing constancy of "half-wave potential" of Cd2+ ion reduction at different concentrations of CdCl2. |
Ilkovic further completed the basic theory of polarography by deriving and verifying an equation for the double-layer charging current at the dropping mercury electrode. That made polarographers aware of the capacity of the electrode double layer and allowed them to follow adsorption of the solution components at the mercury surface. The charging current, which is basically inherent in polarographic measurements, has an average value of the order of diffusion currents corresponding to about 10 µM concentration of an electroactive substance. Thus, it limits to this concentration the sensitivity of simple polarographic measurements, at lower concentrations the diffusion current is overlapped by higher charging current. As the mean charging current, unlike the diffusion current, increases linearly with applied voltage, Ilkovic with Semerano suggested sending through the galvanometer an empirically selected artificial counter-current decreasing with applied voltage, which would thus compensate to some extent the unwanted charging current. Since then, the Ilkovic-Semerano circuit is included in commercially produced polarographs.
When an electroactive substance is present in the solution, it manifests itself on the polarographic curve as a stepwise increase of current, the shape of which can be regarded as a wave. The height of the step, or of the wave, is the limiting current, a measure of concentration of the substance in the solution. For quantitative analytical measurements it is not necessary to record the whole curve, it is sufficient to apply to the electrode a constant potential, within the range of the limiting current, and to measure the change of the limiting current as function of concentration in the course of a titration. Such titrations, originally called "polarometric", have later become known as "amperometric" titrations.
In order to derive an equation for the whole polarographic wave, Heyrovsky and Ilkovic considered the simple common case of an electroactive substance transported in the solution towards the electrode by diffusion, and undergoing a fast and reversible exchange of electrons with the electrode. Then, by combination of the Nernst and the Ilkovic equations one arrives at the Heyrovsky-Ilkovic equation of the diffusion-controlled polarographic wave. The potential, at which the current reaches one half of the total waveheight, has been called the half-wave potential. For a reversible redox-system the half-wave potential, denoted as "E½", is constant, independent of concentration (Figure 7). Values of "E½" of various electroactive substances have been since listed in many textbooks and manuals; "E½"has become an important symbol in electrode kinetics.
Apart from diffusion currents, also a different type of polarographic current was observed: as a rule, this current is much higher than the diffusion current, it appears in the negative range of potentials, it strongly depends on pH of the supporting solution, and it occurs with compounds which are not necessarily electroactive, like proteins or alkaloids. This current was explained as due to catalytic evolution of hydrogen at the mercury electrode: the high hydrogen overpotential of mercury becomes lowered when an apt substance, the catalyst, mediates transfer of hydrogen ions from solution to close contact with negatively polarized mercury surface, where they undergo reduction to gaseous hydrogen. A great number of electroinactive substances can thus be followed polarographically. Heyrovsks's second assistant, Dr. Rudolf Brdicka, discovered a sensitive catalytic hydrogen-evolution reaction of proteins: in buffer solutions of pH about 9, containing ions of cobalt, proteins yield a prominent catalytic "double-wave"; this polarographic reaction was used in many countries over several decades as a diagnostic tool in treatment of cancer (Figure 8).
 Polarography becomes a standard technique
Polarography becomes a standard technique
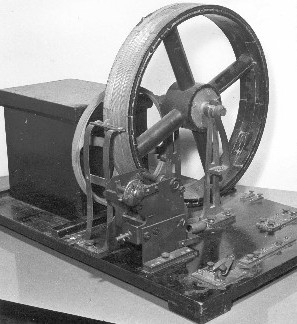 |
| Fig. 9. The first polarograph built at the University, which became a model for commercial instruments. |
After the success of the first polarographs made in the mechanical workshop of Department of Physics at the Faculty of Science (Figure 9), a commercial production of the photorecording instruments was started in Prague in 1929; in the following years various foreign-made polarographs were appearing on the world market, indicating growing interest in the method. With the intention of making the results of Czechoslovak chemists known abroad, Heyrovsky and his senior friend, Professor Emil Votocek from the Prague Technical University, started in 1929 the monthly journal "Collection of Czechoslovak Chemical Communications", which accepted papers in English and French; the "Collection" kept publishing all important results of the Prague polarographic school. Dissemination of polarography in USA was initiated in 1933 by Heyrovsky, who, as Carnegie Visiting Professor, lectured for five months at universities in California and in the middle and eastern States. In German speaking countries, polarography became first known from a chapter by Heyrovsky, included in 1936 in the second volume of the well known series "Physikalische Methoden der analytischen Chemie", edited by W. Bottger from Leipzig University. The interest in polarography in Soviet Union was spurred by the Russian geochemist A. P. Vernadskii, who sent his coworkers to learn the method in Prague, he also had Heyrovsky's Czech textbook from 1933 translated into Russian, and he invited Heyrovsky to lecture on polarography on the occasion of Mendeleev's 1934 centenary celebrations in Russia. The main pioneer and teacher of polarography in the USA over several decades was Professor Isaak Maurits Kolthoff, an analytical chemist at the University of Minneapolis, Minnesota, he visited Heyrovsky's laboratory several times in the nineteen-thirties, he became interested in the method and wrote a well known textbook on polarography which appeared in two editions and was translated into other languages. The French chemist Dr. Edgar Verdier spent several months in Prague before the outbreak of World War II doing research in polarography; after returning home he wrote the first French textbook on the subject. Thus the knowledge and use of polarography in the world was spreading – while by the end of 1930 the polarographic bibliography contained a total number of 125 publications, after ten years it was already at 900.
Ilkovic's work in building the polarographic theory was continued by Rudolf Brdicka. He provided a general treatment of a two-stage reversible polarographic reduction with formation of semiquinones and their dimers, he introduced the concept of polarographic currents controlled by adsorption of the electroactive species, and, with his junior coworker Karel Wiesner, he opened the wide field of polarographic currents controlled by chemical reactions. The dropping mercury electrode was thus supplying new topics for general electrode kinetics.
During World War II, polarography, as a modern analytical technique, was applied in various branches of the war industry including the development of the atomic bomb – in the "Manhattan Project" the transuranium elements were determined by several polarographs. Czechoslovakia was occupied by the German army, and the Czech universities were taken over by Germans. Thanks to the help of his anti-nazi colleague, German physical chemist Professor Johann Bohm, Heyrovsky was able to continue working in his laboratory. He used this time, free of teaching duties, to finish his major textbook on polarography, which appeared in German published by Springer in Vienna, and he began to use cathode-ray oscilloscope in polarographic research. Complying with the possibilities of electronic technology at that time, and intending to try another type of electrolytic polarization, he introduced what he called "oscillographic polarography". The dropping mercury electrode was polarized by alternating current and the corresponding rapid changes of electrode potential were followed on the screen of an oscilloscope. That method allowed the study of fast electrode processes; for that purpose, Heyrovsky, with his coworker Dr. Jindrich Forejt, was using a mercury jet electrode, with which the contact between the electrode surface and the solution lasted only fractions of a second. For analytical purposes the potential dependence of the first derivative of electrode potential by time was used – it formed a closed, oval-shaped curve, with cathodic and anodic branches opposite each other; on such a curve, the electrolytic processes appeared as indentations. That technique clearly displayed reversibility of the processes involved, and accelerated polarographic analysis from minutes to seconds.
In the years after the war, polarography was among the five most often used methods of chemical analysis. As a guest of the British Council in 1946, Heyrovsky visited several universities in the UK, lecturing on polarography. The next year he was invited by Svenska Institutet to lecture in Sweden and in Denmark. The first international congress on polarography was organized in Prague in February of 1951. By that time, Czechoslovakia had become part of the Soviet bloc and potential participants from abroad were not admitted to the country; however, most of them submitted their contributions to the congress proceedings, which appeared in three volumes. The organizer of the congress was the "Institute of Polarography", one of eight new institutes founded in 1950 by the communist government in order to boost research in science and technology. Heyrovsky was appointed director of the institute, nevertheless, as he was not politically trusted by the regime, his further invitations to conferences and to lecture tours abroad were being turned down without official reason. When the new Academy of Sciences, according to Soviet example, was established in the country in 1953, the Institute of Polarography was included in it, and Heyrovsky, as institute director, was nominated for membership in the Academy. His teaching duties at the University were reduced, and the directorship of the Department of Physical Chemistry was taken over by Brdicka, who had in the meantime become full professor.
The task of the newly established academic Institute was to carry out basic research in all fields of polarography, to introduce and supervise its practical applications in the country, to collect and publish polarographic bibliography, and to control the state-organized production of polarographs. This sounded very good, however, the world situation was not very favorable for such a project. Because of political division of the world, and due to forced isolation of Czech experts from their western colleagues, the progress of technology in the entire eastern bloc, especially in the field of electronics, was lagging behind. The technical progress of the post-war world required highly sensitive, specific, and rapid methods of analysis. The original, simple form of polarography needed further modifications to meet these requirements. From the nineteen-fifties on, the innovations and improvements of polarographic instrumentation were steadily moving to western countries together with the advanced theoretical studies brought about by the new techniques. In this way, the Prague polarographic school was gradually losing its exclusive position in instrumental and theoretical polarography.
Members of the Heyrovsky's group tried to keep pace with the international development. Another Heyrovsky's pre-war assistant at the University, Dr. Mirko Kalousek, introduced the "commuter polarography", in which, in course of recording the curve, the potential of the dropping electrode could be commuted with variable frequency to a certain selected constant potential; the resulting curve could then indicate reversibility of the studied electrode reaction, and electroactivity and other properties of its prospective intermediates. Shortly after the war, Heyrovsky's junior coworker, electronic engineer Augustin Sevcik, began applying linear voltage pulses of large amplitudes to slowly growing mercury drops and observed the resulting curves on the screen of an oscilloscope – that was the "single-sweep" or "multi-sweep" oscillographic polarography, according to whether one or several voltage pulses were applied to one drop, and one or more current-voltage curves appeared on the screen of the oscilloscope. Instead of the polarographic waves, the curves were peak shaped, for which Sevcik derived a corresponding equation; as the method and equation were published independently at the same time by A. Sevcik in Prague and by Professor J. E. B. Randles in Birmingham, the mathematical formulation of the corresponding current-potential curve is known as the Randles-Sevcik equation.
 |
| Fig. 10. Oscilloscope displaying an "a.c. oscillopolarographic" curve obtained with a mercury-jet electrode. In the background picture of professor B. Kucera. |
In addition to the original, or "dc", polarography, Professor Bruno Breyer in Australia introduced "ac" polarography (Figure 10), in which small amplitude ac voltage was superimposed on the dc voltage applied to the dropping electrode, and, instead of direct current, the ensuing alternating current was measured, as function of the applied mean dc voltage. Such curves were characterized by maxima, or peaks, in place of half-wave potentials of dc polarography. Prominent peaks appeared on the curves also when the electrode capacity was changed by fast adsorption or desorption of a surface-active compound. Later, placed on a more rigorous basis, two different alternating currents have been recorded separately – that flowing in-phase with the applied alternating voltage, and that flowing out-of-phase with it. In that way the complex impedance of the dropping electrode is measured which provides exact data for electrode kinetics in general, and for study of adsorption processes in particular.
Similar to ac polarography, though much more sophisticated in detail, is the "square-wave polarography", developed by Dr. Geoffrey Cecil Barker in the Atomic Energy Research Establishment at Harwell, UK. Instead of the usual sine-wave shape, the alternating voltage has here a basically rectangular form and the current is measured during the short intervals before each change of the rectangular voltage half-period. This way, the charging current has been eliminated to a large extent, and the sensitivity of polarographic determinations was increased by about two orders of magnitude of concentration in the solution. Barker's laboratory became a rich source of further development of polarographic instrumentation. Pulse polarography, and especially its differential version, brought the sensitivity of polarographic analysis to its limit – in these methods, a single potential pulse is applied to each mercury drop, and the current, or the difference of currents in the differential version, is measured before the end of drop-time, essentially eliminating the charging current component. "Radio frequency polarography" and "Intermodulation polarography" have been introduced for solving problems in advanced electrode kinetics. Barker combined all his highly complex electronic techniques solely with the dropping mercury electrode, as he trusted its exquisite properties as a scientific tool.
In the nineteen-fifties, several polarographers – Drs. Vladimir Cermak and Jir� Vogel in Prague, and Dr. Zenon Kublik and Professor Wiktor Kemula in Warsaw, started using the hanging mercury drop electrode instead of the dropping mercury electrode. That meant that they recorded the whole curve with one single drop of mercury (like in the single-sweep polarography); after the curve was completed, the used drop was knocked off and a new one was produced from the capillary. As this time a stationary electrode has been used, according to the official IUPAC nomenclature the method thus introduced is not "polarography" any more, it has to be called "voltammetry". The curves are still reproducible, however, unlike the dropping electrode, the hanging drop carries its whole "history" along. This simple fact has been utilized to produce a considerable increase of sensitivity in electroanalysis: using a selected constant potential and controlled stirring, a product of the electrolytic process is quantitatively accumulated at the electrode surface, either in the form of an amalgam or in the form of an adsorbed layer; after an exactly measured time of electrolysis, a linear voltage scan is applied and the current-voltage curve is recorded, displaying the electrode reaction in reverse. This voltammetric operation is known as "stripping", of which three modifications are distinguished: anodic stripping (usually dissolution of a metal from the previously formed amalgam), cathodic stripping (usually reduction of compounds with mercury, previously formed at positive potentials) and adsorptive stripping (usually desorption of species previously accumulated by controlled adsorption). The voltammetric stripping methods allow analytical determinations of species in dilutions as low as one tenth of one billionth molar concentration (M) and lower. Professor Emil Palecek developed the "adsorption transfer voltammetric stripping" in 1986 for electroanalysis of biological macromolecules – in this technique, rare substances are accumulated by adsorption from a minute volume (millionth of a liter) of solution to the surface of hanging mercury drop; that electrode with the adsorbed layer is then transferred to a normal cell with usual volume of suitable electrolyte, for a proper study by some of the stripping techniques. The G. C. Barker's instrumental techniques developed for the dropping electrode can be, and very often are, used with the hanging mercury drop electrode, thus, for example, the very limits of analytical sensitivity can be reached with the differential pulse adsorptive, cathodic or anodic, stripping voltammetry.
 The Nobel Prize
The Nobel Prize
 |
| Fig. 11. Jaroslav Heyrovsky having received the Nobel Prize from the Swedish king. |
The second international congress on polarography took place in August of 1959 at Cambridge, UK. A group of polarographers from Czechoslovakia was allowed by the communist authorities to participate; unfortunately, Jaroslav Heyrovsky could not attend because of ill health. The proceedings of the congress were published by Pergamon Press in three volumes. In October of the same year, Heyrovsky received an official announcement from the Royal Swedish Academy of Sciences that he would be awarded that year's Nobel Prize in Chemistry "for his discovery and development of the polarographic methods of analysis". Accompanied by his wife (the two children were not allowed to leave the communist-ruled country) Heyrovsky went to Stockholm to receive the golden medal and the diploma from the hands of the Swedish king, Gustav Adolph VI, in a solemn ceremony on the 10th of December (Figure 11). That was the supreme recognition of his dedication to scientific goals, and, at the same time, the recognition of the importance of the polarographic method. Heyrovsky's only comment on the official formulation of the text in the diploma was that polarography is in principle a physico-chemical method of which the analytical application is just one aspect.
Possibly as an aftereffect of the Nobel Prize for polarography, three international polarographic congresses were organized in the nineteen-sixties. In 1964 it was in Southampton, UK – two volumes of proceedings were published by Macmillan under the title "Polarography 1964". To celebrate its centenary in 1966, the Czechoslovak Chemical Society convoked the 4th International Congress on Polarography to Prague; the contributions had been included in a special, December 1965, issue of the "Collection of Czechoslovak Chemical Communications", published in honor of Jaroslav Heyrovsky's 75th birthday. In the autumn of the same year, The Polarographic Society of Japan invited polarographers to Kyoto to participate in the 5th International Congress of Polarography; the papers presented on that occasion were published in a special combined issue of the Japanese "Review of Polarography".
In 1964, in view of his declining health, Heyrovsky resigned from the directorship of the Polarographic Institute. He still continued coming daily to his laboratory/office almost to his last day, attending to correspondence, participating in polarographic colloquia, and following recent polarographic literature. After about two-weeks' stay in a Prague state hospital, he passed away on the 27th of March 1967. However, the ideas to which he dedicated his active career, utilization of ideally renewable mercury electrodes for exact measurements, continue living as part of the universal scientific heritage.
 After Heyrovsky
After Heyrovsky
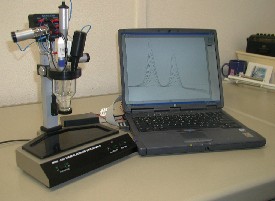 |
| Fig. 12. One of polarographic computer-operated instruments. |
In the second half of the twentieth century, polarography with its innovations was well-established in physico-chemical and analytical laboratories, polarographic instrumentation was available on the market with a variety of accessories, polarographic papers were presented on many national and international seminars and conferences, and the yearly increment of polarographic publications became more or less stabilized at about 500 per year. In 1980, the well attended J. Heyrovsky Memorial Congress on Polarography took place in Prague. In 1990, in the same place, the International Society of Electrochemistry organized its 41st Meeting jointly with the J. Heyrovsky Centennial Congress on Polarography. The latter congress, after the recent fall of the Iron Curtain, was a truly international event. It became clear from the presented communications that polarography and voltammetry, like other instrumental methods at that time, were undergoing a "computer revolution". The sophisticated specific electronic equipments were giving way to a computer with appropriate software, connected to a simple sensor device.
At the beginning of the third millennium, a great variety of software programs can be used in computer-controlled electrochemical instruments to carry out exacting research of problems reaching from material science to biophysics, based on renewed mercury electrodes (Figure 12).
In December 2009, the international conference "Modern Electroanalytical Methods 2009" was held at Prague University to commemorate the 50th anniversary of the Nobel Prize for polarography. The attendance of the conference confirmed that the method in its actual state of development was alive and well.
Related articles
Pillars of modern electrochemistryWalther Nernst: physicist and chemist
Julius Tafel - his life and science
The Electrochemical Society: The First Hundred Years, 1902 - 2002
Volta and the "Pile"
The past, present, and future of electroanalytical chemistry
Bibliography
- Analytical Electrochemistry (2nd edition), J. Wang, Wiley-VCH , New York, 2000.
- Modern Polarographic Methods in Analytical Chemistry, A. M. Bond, Marcel Dekker, New York, 1980.
- Principles of Polarography, J. Heyrovsky and J. Kuta, Academic Press, New York, 1966.
- Polarographic Techniques (2nd edition), L. Meites, Interscience, New York, 1965.
- Modern Polarographic Methods, H. Schmidt and M. von Stackelberg, Academic Press, New York, 1963.
- Polarography (two volumes, 2nd edition), I. M. Kolthoff and J. J. Lingane, Interscience, New York, 1952.
- Researches with the Dropping Mercury Cathode, Part I. General Introduction, Part II. The Polarograph, Part III. A Theory of Over-potential, J. Heyrovsky (and M. Shikata, Part II), �Recueil des Travaux Chimiques des Pays-Bas� Vol. 44 (Ser. 4, Vol. 6), pp 488-495, 496-498, and 499-502, 1925.
Available on the WWW.
- Electrolysis with a Dropping Mercury Catode. Part I. Deposition of Alkali and Alkaline Earth Metals, J. Heyrovsky, �The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science� Ser. 6, Vol. 45, pp 303-315, 1923.
Available on the WWW.
- Elektrolysa se rtufovou kapkovuo kathodou, J. Heyrovsky, �Chemicke Listy� Vol. 16, pp 256-264, 1922. Available on the WWW.
Listings of electrochemistry books, review chapters, proceedings volumes, and full text of some historical publications are also available in the Electrochemistry Science and Technology Information Resource (ESTIR). (http://knowledge.electrochem.org/estir/)
Return to: Top – Encyclopedia Home Page – Table of Contents – Author Index – Subject Index – Search – Dictionary – ESTIR Home Page – ECS Home Page
ECS | Redcat Blog | ECS Digital Library