Return to:
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
ELECTROCHEMISTRY FOR K-12: THE POTATO CLOCK AND BEYOND
Ann Abraham,1 Attila Palencsar,2 and Daniel Scherson2
1Department of Chemistry, Kent State University, Ashtabula Campus
Ashtabula, OH 44004, USA
E-mail: aabraha3@kent.edu
2Department of Chemistry, Case Western Reserve University
Cleveland, OH 44106, USA
E-mail: dxs16@cwru.edu
(August, 2007)
 |
Hybrid vehicles, fuel cells, and,
particularly, market demands
for longer lasting batteries to
power portable communication and
entertainment devices have propelled
electrochemistry to the forefront of news media and advertisement. Despite this
welcome awakening, an understanding
of the fundamental principles that govern
this highly multidisciplinary field
by the public at large appears to be lacking.
This state of affairs can be traced,
at least in part, to our K-12 educational
system, which fails, in great measure, to
introduce electrochemistry as part of the
curriculum. Regrettably, a golden opportunity
to capitalize on batteries and rust,
terms familiar to children very early in
life and precious sources of experiential
learning, is irrevocably lost. It thus
becomes of pressing interest to explore
possible avenues to remedy this situation
and bring awareness to the next
generation of the importance of electrochemistry
as a scientific and technical
discipline and its impact on energy and
the environment.
The challenges we face as a community
toward accomplishing this laudable
goal are by no means minor. Glamorous
topics, such as space and dinosaurs, have
captured the minds not only of children
but their parents as well, through
the Hollywood industry and its glitz.
Nothing of this sort can be said about
electrochemistry. Our field continues to
hide behind a perceived lack of luster,
exacerbated by the unfortunate paucity
of eye-catching, adrenaline-powered
experiments and classroom material with
which to engage such fierce and formidable
attention-seeking competitors.
Even if these seemingly insurmountable
hurdles are overcome, it still remains a
delicate pedagogical duty for educators
to maximize conceptual simplicity, without
compromising scientific accuracy.
This article seeks to prospect the
problem and trace a possible road map
toward achieving the desired educational
aims. We review as a starting point, the
recommendations of the National Science
Education Standards (hereinafter, "the
Standards") that bear relevance to electrochemistry,
and highlight the ways in
which electrochemical concepts are gradually
introduced through the various
stages of schooling. Later, we critically
assess the pedagogical value and scientific
accuracy of selected books and videos
available to K-12 educators and students,
and, finally, we share our personal field
experiences teaching electrochemistry
through experiments to a younger
audience.
National Science Education Standards
The website for the Standards (http://www.nap.edu/readingroom/books/nses/html)
provides guidelines for achieving scientific
literacy through the K-12 educational
system. The sections in italics below
are excerpts with relevance to electrochemistry
extracted from that document.
 K-4 grades: K-4 grades:
" In most children's
minds, electricity begins at a source and
goes to a target. This mental model can be
seen in students' first attempts to light a
bulb using a battery and wire by attaching
one wire to a bulb. Repeated activities will
help students develop an idea of a circuit
late in this grade range and begin to grasp
the effect of more than one battery. ...
Electricity in circuits can produce light, heat,
sound, and magnetic effects. Electrical circuits
require a complete loop through which
an electrical current can pass".
At this early stage of education, batteries
are introduced within the context
of electricity, as devices which can power
other devices, for example, lighting a
bulb. Utility takes priority over principles
of operation – emphasis is placed
on what batteries can do, NOT on how
batteries work.
 5-8 grades: 5-8 grades:
" Chemical elements do not
break down during normal laboratory reactions
involving such treatments as heating,
exposure to electric current, or reaction with
acids. There are more than 100 known elements
that combine in a multitude of ways
to produce compounds, which account for
the living and nonliving substances that we
encounter. ... Electrical circuits provide a
means of transferring electrical energy when
heat, light, sound, and chemical changes
are produced. In most chemical and nuclear
reactions, energy is transferred into or out of
a system. Heat, light, mechanical motion,
or electricity might all be involved in such
transfers".
In this intermediate stage, electricity
and chemistry become intertwined.
Electricity provides a means of doing
chemistry. General aspects of energy
conversion are first introduced.
 9-12 grades: 9-12 grades:
" When students observe
and integrate a wide variety of evidence,
such as seeing copper "dissolved" by an
acid into a solution and then retrieved as
pure copper when it is displaced by zinc, the
idea that copper atoms are the same for any
copper object begins to make sense. In each
of these reactions, the knowledge that the
mass of the substance does not change can
be interpreted by assuming that the number
of particles does not change during their
rearrangement in the reaction. ... A large
number of important reactions involve the
transfer of either electrons (oxidation/reduction
reactions), or hydrogen ions (acid/base
reactions) between reacting ions, molecules,
or atoms. In other reactions, chemical bonds
are broken by heat or light to form very
reactive radicals with electrons ready to
form new bonds".
The last four years of basic education
address redox chemistry per se, although
the term electrochemistry is not being
mentioned explicitly. Redox reactions
are a kind of chemical reaction. It must
be recognized that chemistry at this
stage is only a part of their science academic
requirements, which also includes
physics and biology. It is our view that
at this stage, electrochemistry should
be used to illustrate the commonality
of processes, which to the K-12 student
would seem utterly disparate, such as
respiration and photosynthesis and the
operation of fuel cells and Graetzel's dyesensitized
photoelectrochemical cells.
Nature is governed by only a surprisingly
small set of rules, a concept that permeates
college science education.
What are they teaching our children?
Advantage can be taken within
the framework of the Standards, to
design well-conceived experiments and
graphic material, as vehicles to instill
electrochemical curiosity among the
young. Aware of this potential market,
a few commercial enterprises have
produced attractive educational videos,
books, and kits. A few Sunday afternoon
journeys to the local bookstores, coupled
with several hours of browsing the
Web, unveiled a number of surprising
and often puzzling results. Although
by no means exhaustive, some of
our most interesting findings of the
extent to which electrochemistry is
presented as a scientific discipline, and
the wide spectrum of the quality of the
information available are summarized
next.
Video material – a great idea!
 |
| Fig. 1. © North America Syndicate. Reproduced by permission.
|
It was exciting to find a set of
commercial educational videos devoted
solely to electrochemistry, and be able
to examine their content free of charge
thanks to the kind willingness of the
distributor, http://www.films.com. This series consists of six, 10 min modules: Building
Blocks of Electrochemistry, Electrochemical
Cells, Designing Electrochemical Cells,
Commercial Electrochemical Cells,
Corrosion, and Electroplating. The choice
of topics is highly appropriate, as it
capitalizes on everyone's familiarity with
batteries (as electrochemical devices) and
corrosion (as an electrochemical process)
to introduce some of the underlying
principles involved. Somewhat
charming, at least initially, was the
introduction of a robot as the central
character, equipped with a battery
heart and a metallic body particularly
prone to corrosion when exposed to
the salty tear drops of the (non-robot)
woman of his dreams. Although such
an organic-inorganic amorous liaison
may well be effective in grasping the
short attention span of the youngest
viewers, the degree of complexity of
the more serious subject matter is
definitely targeted toward a much older
audience, who may regard the motif as
too childish and thus beneath them. It
did not remain unnoticed, at the outset,
that about 10% of the precious time
was just filler, and, also, that segments
of the technical material were repeated
in many of the modules. A sizable
amount of corrosion was included in the
electrodeposition module. Nevertheless,
and with only a few exceptions, most of
the material presented was technically
correct. Particularly noteworthy was
the emphasis placed on both positive
and negative ions as responsible for
electrical conduction in the electrolyte
solution (for which the concentrations
are forgivably expressed in highly
unconventional kmol/m3 units), as well
as on corrosion, as being a two-redox
reaction process. The introduction
to cathodic protection was especially
well delivered. Also welcome were
analogies to common experiences, such
as a pitcher-catcher duet representing
oxidation-reduction, with the baseball
playing the role of the electron. In stark
contrast, attention to detail was lacking,
ranging from the anachronic (such as an
original Volta pile powering a light bulb)
to the outdated (a Leclanche battery
as a model energy storage device, or a
museum-type voltmeter displaying a
needle that flickered mystifyingly when
connected to an electrochemical cell).
A cursory review by expert consultants
would have uncovered far more serious
errors, such as referring to the release
of electrons by zinc metal as a reduction,
the generation of hydrogen atoms in the
electrolyte solution, and depicting the
role of the separator as the element that
prevents contact of the anode with the
electrolyte.
Printed material – is anyone watching?
Our pilgrimage through the limited
array of electrochemically-related material
available at the local national chain
bookstore outlets was eventful. In contrast
with the very small selection of science
resources for children, and the few
paragraphs devoted to electrochemistry
within those sources, the conceptual
electrochemical errors we found, without
looking very hard, were comparatively
many and highly disconcerting. A brief,
and by no means comprehensive, selection
of errors included: "All batteries
contain two electrodes and an electrolyte,
which produces the chemical reaction
with the electrodes resulting in a
current;" "In 'dry' batteries, the electrolyte
is a paste of powdered chemicals;"
and "A car battery produces a strong
current by using a number of cells linked
together". Furthermore, while explaining
the principle of operation of a lemon
battery, another publication states, in
one case, that "The lemon juice will
take electrodes (although probably the
authors meant electrons) from the copper
and transfer them to the iron creating
an electric current"; and in another,
involving copper and zinc electrodes, that
"The experiment creates an electric current
of almost 1 volt"!
Experiences in the field
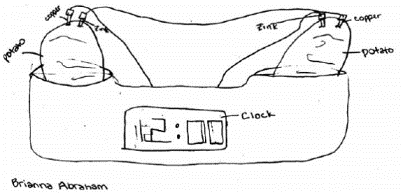 |
| Fig. 2. Potato clock experiment in progress. Drawing © Brianna Abraham, reproduced with permission. |
One of the authors (A.A.), a parent of
school age children and a Ph.D. in organic
chemistry, has had an opportunity to
teach "Kid Chemistry" to K-8 graders
as a volunteer for the past eight years.
Intrigued by the uncertainties of the outcome,
she used students as the subject of
an experiment aimed at assessing their
reaction when exposed to electrochemistry-related materials. Following a thorough search in science and educational
supply catalogues, she encountered the
Two Potato Clock™, a kit (http://www.starmagic.com/) that incorporates a
digital clock that runs on two fresh
potatoes (not included) hooked together
by three metal wires (included). In
addition to the plastic container, the
kit included two zinc and two copper
electrodes attached to the ends of the
wires. Students were asked to follow the
instructions provided with the kit and
to draw a diagram of the setup (Figure 2).
Surprisingly, the more diligent students
invariably failed to make the clock work.
Far more successful was the plug-and-play
cohort of students, who had not
bothered to read the instructions that
had erroneously directed them to "insert
the copper probe end ... into the first
potato with the copper probe ...", but
who simply tried all possible combinations.
After all, electrochemistry (as with
chemistry in general) is an experimental
science! This experience illustrates
the two-track approach to education:
do and learn and/or learn and do, as
underscored by the Standards: "'Hands-on'
activities, while essential, are not
enough. Students must have 'minds-on'
experiences as well". The harmonious
interplay between theory and experiments
is the key to research progress, as
we professional scientists already know
and practice.
Incidentally, the ball now is in the
reader's court to figure out (without
looking at the instructions) how to wire
the potatoes correctly, and the nature
of the electrochemical processes responsible
for the battery operation. So, write
down the balanced half-cell reactions,
and with the help of suitable tables,
make some predictions as to the cell
voltage the device should display. As a
hint, other nourishing and non-nourishing
products, such as tomatoes, lemons,
and cola, also work. We will not divulge
at this juncture whether a single, as
opposed to the suggested two potatoes
in series, may be sufficient to run the
clock. Nevertheless, you are invited to
report back to us at yces@yces.case.edu.
Beyond the potato clock
 |
| Fig. 3. Conductivity experiment in progress. Middle scientist is testing solution in well-plate with a blinking LED indicator. Test solutions in dropper bottles and data sheet are also shown. |
In yet another experiment (Figure 3),
students were given a variety of relatively
inexpensive conductivity indicators
available in the educational catalogues,
including a ringing bell of variable
intensity and pitch, and two different
blinking LEDs (red light) of variable
intensity and frequency (http://www.sciencekit.com;
http://www.lab-aids.com).
Students (4-6 graders) received a
set of solutions of acids and bases, and
a salt, of various concentrations, and
well water, orange juice, milk, and a few
soft drinks, together with two different
types of indicators and a data sheet.
Students were encouraged to use their
own words to describe the outcome of
their experiments, in terms of what they
heard or saw. Once the initial thrill of
listening to the ringing bell had quelled,
students, appropriately classified, with
little guidance, the responses obtained
with the various solutions, as loud,
medium, no sound, extra loud, and soft.
Similar, descriptive words were used to
explain the results observed with the
blinking LED indicators, e.g., consistent
blinking, doesn't blink, faint light, bright
light, very faint light, very very faint
light, and blinking rapidly. One group,
in an attempt to make the response of a
qualitative indicator more quantitative,
tried to record the number of blinks
in 10 s. A range of 0 to 13 blinks was
recorded for the various samples.
Overall, the quantitative indicators
(those showing actual numbers) were
preferred by the students (an informal
poll was conducted at the end of the
experiment) because the response
needed only a single number on the data
sheet. As intended, the different types
of indicators allowed students to match
qualitative results with quantitative data.
Both the potato clock and the conductivity
indicators fall within the
Standards guidelines. In particular, both
the conductivity measurements and the
potato battery involve a complete loop
through which an electrical current can
pass, or circuit. In addition, they show
that circuits (when wired properly) can
be used to run a clock, elicit sounds,
or generate light as in the conductivity
experiments (K-4). Furthermore, the
potato clock could, although no such
experiment was performed, help students
grasp the effect of more than one
battery. They also illustrate, as suggested
for grades 5-8, that electrical circuits
provide a means of transferring electrical
energy when heat, light, sound, and
chemical changes are produced.
The teenage years
As was mentioned earlier, K-8 should
set the stage for introducing in grades
9-12 the principles underlying the, by
then familiar, electrochemically oriented
experiments through experiential
learning. Although not as yet tested
by the authors in the field, commercial
vendors have been identified who
offer a variety of equipment suitable to
meet the high school Standards. These
include electrodeposition kits, which
could serve to illustrate that the same
metals atoms can be part of a solid or
dissolve in a solution in a reversible
fashion. Particularly interesting is a
small car that incorporates an array of
solar cells, which upon illumination
generate sufficient power to electrolyze
water to produce hydrogen and oxygen
gas (http://www.fuelcellstore.com/).
In a second stage, these gases are fed to
the appropriate electrodes in a fuel cell,
which then serves to power an electric
motor that sets the car in motion. This
ingenious instructional toy provides
numerous opportunities to explore issues
of energy conversion and storage and
perhaps, with a bit more effort, introduce
the concept of conversion efficiencies.
Pedagogical links to biology can be
readily introduced using the cathode of
the fuel cell as a breathing component,
in that it mimics respiration which
involves the enzymatic reduction of oxygen
to yield water.
Yet another equally ingenious kit
(http://www.solideas.com/solrcell/cellkit.html)
provides glass plates coated first
with a transparent conductive layer
(tin oxide), and then a porous titanium
dioxide film, which is soaked into either
freshly crushed raspberries or blackberries,
pomegranate seeds mixed with a
tablespoon of water, or red hibiscus tea
(not included). A drop or two of a premade
iodide/iodine solution (included)
is then placed on the stained portion of
the film to serve as the (redox active)
electrolyte in the solar cell to complete
the circuit. The cell is closed by placing
a second tin oxide coated glass piece
(counter electrode) on the porous film.
Once assembled and exposed to full sun
through the titanium dioxide side, the
cell outputs approximately 0.43 V of voltage and 1
mA/cm2 current density. This device is capable of using
light to carry out a redox reaction and,
as such, shares common features with
photosynthesis, which fundamentally is
an electrochemical process.
We close this brief article by supporting
wholeheartedly the National
Science Education Standards general
view that:
"No one group can implement
the Standards. The challenge extends to
everyone within the education system,
including teachers, administrators, science
teacher educators, curriculum
designers, assessment specialists, local
school boards, state departments of education,
and the federal government. It
also extends to all those outside the system
who have an influence on science
education, including students, parents,
scientists, engineers, businesspeople,
taxpayers, legislators, and other public
officials. All of these individuals have
unique and complementary roles to play
in improving the education that we provide
to our children."
Acknowledgement
This article was reproduced from The Electrochemical Society Interface (Vol. 15, No. 3, Fall 2006) with permission of The Electrochemical Society, Inc. and the authors.
Listings of electrochemistry books, review chapters, proceedings volumes, and full text of some historical publications are also available in the Electrochemistry Science and Technology Information Resource (ESTIR). (http://knowledge.electrochem.org/estir/)
Return to:
Top –
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
|



