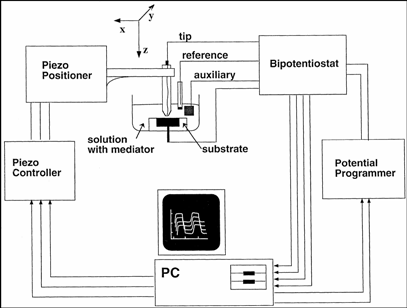
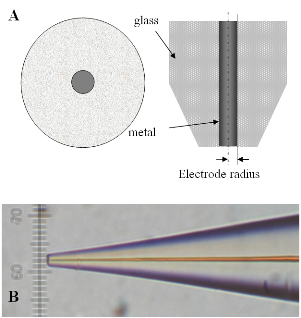
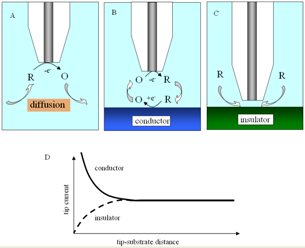
While the tip is far from the substrate (Figure 3A) the faradaic current at the tip generated by this reaction is constant and limited by the speed at which "R" diffuses toward the electrode. When the tip is brought to within a few tip radii of a conductive substrate surface (Figure 3B), the "O" species formed at the tip diffuses to the substrate, where it can be instantly reduced back to "R". This cycling increases the flux of "R" to the tip and hence creates a "positive feedback", that is, the tip-current increases. The closer the tip is to the substrate, the larger the tip-current.
If the substrate is an electrical insulator (for example, glass), the tip-generated
species, "O", cannot react at its surface. In this case "R" is not regenerated by
the substrate and its diffusion toward this tip is hindered. The tip-current
decreases as the tip approaches the substrate ("negative feedback"; Figure 3C).
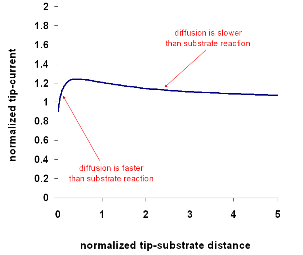
Using the well-developed quantitative theory for each situation, one can
simulate tip-current against distance curves with the help of computers. A fit
between experimental and numerical data will give quantitative information about
the rate of the regeneration reactiom.
|
||||||||||||||||||||||||||||||
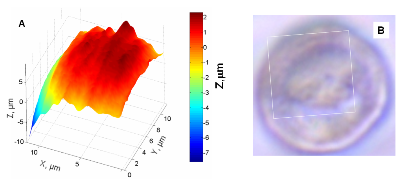
 |
| Fig. 5. Substrate imaging. (A) Tip is maintained at constant height while scanning horizontally. (B) SECM image of a portion (1 µm × 1 µm) of a human breast cell membrane acquired with a 47 nm radius ultramicroelectrode. |
 Chemical reactivity
Chemical reactivity
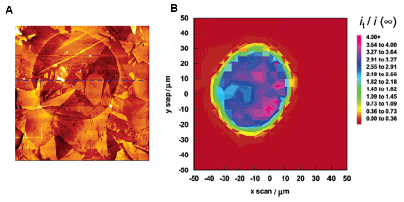 |
| Fig. 7. (A) 80 µm × 80 µm atomic force microscopy image of a disk-like region of boron-doped diamond. (B) SECM image over such a region. |
Some applications of SECM
 Measurement of rates of reactions at interfaces
Measurement of rates of reactions at interfaces
The kinetics of reactions at the interface between a solid and a liquid, for example,
metal or semiconductor in solution, can be probed by SECM. The technique
can also be applied to measure kinetics of reactions at the interface between
two immiscible solutions (for example, water/oil). To perform a measurement,
one records the tip-current as the tip is approached toward the interface. The
experimental tip-current versus tip-substrate distance curve is then matched
with computer simulated curves and the value of the speed of reaction occurring
at the interface can be obtained.
 Electrocatalysis
Electrocatalysis
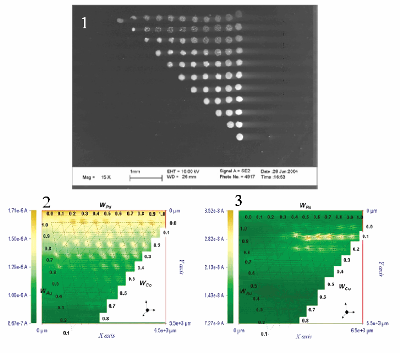 |
| Fig. 8. Array of metal mixture catalytic spots on a glassy carbon substrate. (1) Scanning electron micrograph of the array (rotated 90o clockwise). (2) - (3) SECM images with substrate biased at 0.2 V and 0.75 V respectively against the hydrogen electrode. |
 Redox enzymes
Redox enzymes
 |
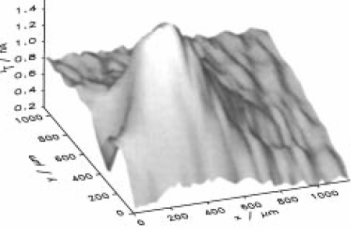 |
| Fig. 9. Optical micrograph of substrate showing dark-colored immobilized enzyme pattern (left). SECM image of the enzyme activity (right). | |
 Corrosion and dissolution
Corrosion and dissolution
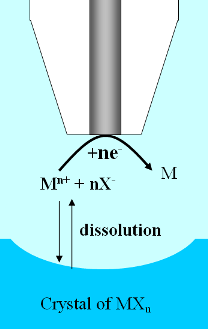 |
| Fig. 10. Scheme of SECM-induced ionic crystal dissolution. |
- detection of small pits and pit precursors, that is, electrochemically active sites on which pits nucleate in the course of an experiment;
- monitoring concentration profiles of the corrosion participants and products; and
- the use of the tip to induce the pit formation.
Dissolution of ionic crystals is a phenomenon that can be studied by SECM. The
ultramicroelectrode tip can deplete a chemical species by reducing or oxidizing it. If the
initial chemical species is in equilibrium with the crystal then the depletion
of the earlier dissolves the crystal as shown in Figure 10. The high spatial
resolution of SECM enabled scientists to determine that the dissolution of
copper sulfate crystals into water occurred at surface defects.
 Surface patterning
Surface patterning
 |
| Fig. 11. (A) Scheme of SECM metal deposition, metallic ions are reduced at the tip and oxidation occurs at the substrate/polymer interface. (B) Scanning electron micrograph picture of a pattern of silver lines deposited in a Nafion film. |
 Membrane transport
Membrane transport
Some scientists have used SECM to investigate the transport of chemicals through
natural or artificial membranes, and biological tissues. SECM seems
to be particularly suited for such studies because in many cases the transport
happens through micro pores in the material and therefore is spatially
constricted. Elucidating transport mechanisms can help understand the
functionalities of biological tissue such as skin, dentine, or cartilage.
 Other applications
Other applications
SECM has been used to probe adsorption and desorption of chemical species on
surfaces. It has been applied to metabolism study of single living cells, other biological systems, and charge transfer mechanism in
conductive polymers, efficiency of photoelectrochemical conversion, etc. These applications of SECM and other are further discussed in the
references below.
 The push for nano
The push for nano
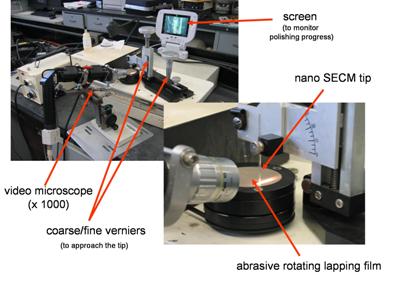 |
| Fig. 12. Nanoelectrodes polishing station. |
- With smaller tip the measurement of the rate of extremely fast redox reactions becomes possible.
- Submicrometer sized electrodes permit high resolution SECM imaging.
As can be expected, the smaller the electrodes the more fragile they become.
Fabricating SECM tip of nanometer dimension is a very challenging task as these
electrodes need to be polished by grinding the tip with abrasive material.
Figure 12 shows a set up for polishing nanometer size ultramicroelectrodes.
Conclusions and prospects
SECM is a valuable and indispensable tool for the study of surface reactivity. Scientists are more and more attracted to this method because of its simplicity of use and the quantitative results it can deliver. The fast expansion of the SECM field during the last several years has been fueled by the introduction of new probes, commercially available instrumentation, and new practical applications. The greatest challenge of SECM will be to routinely do measurements with nanometer-sized electrodes, as these electrodes are much harder to produce and use than micron-sized one. The fabrication of nanometer-sized tips and the development of numerous hybrid techniques have already greatly enhanced the SECM capacity to solve problems in cell biology, surface science, and nanotechnology.Bibliography
- Bibliography for SECM Papers and Closely Related Material, D. O. Wipf, available at www.msstate.edu/dept/Chemistry/dow1/secm/secm_bib.html.
- Physicochemical Applications of Scanning Electrochemical Microscopy, F. O. Laforge, P. Sun, and M. V. Mirkin, in "Advances in Chemical Physics" Vol. 139, pp 177-244, Wiley-Interscience, New York, 2008.
- Scanning Electrochemical Microscopy in the 21st Century, P. Sun, F. O. Laforge, and M. V. Mirkin, "Physical Chemistry Chemical Physics" Vol. 9, pp 802-823, 2007.
- Scanning Electrochemical Microscopy for Direct Imaging of Reaction Rates, G. Wittstock, M. Burchardt, S. E. Pust, Y. Shen, and C. Zhao, "Angewandte Chemie International Edition" Vol. 46, pp 1584-1617, 2007.
- Scanning Electrochemical Microscopy, F.-R. F. Fan, J. Fernandez, B. Liu, and J. Mauzeroll, in "Handbook of Electrochemistry" pp 471-540, C. G. Zoski (editor), Elsevier, Boston, 2007.
- Biological Applications of Scanning Electrochemical Microscopy: Chemical Imaging of Single Living Cells and Beyond, S. Amemiya, J. Guo, H. Xiong, and D. A. Gross, "Analytical and Bioanalytical Chemistry" Vol. 386, pp 1584-1617, 2006.
- Imaging Localized Reactivities of Surfaces by Scanning Electrochemical Microscopy, G. Wittstock, in "Solid-Liquid Interfaces (Topics in Applied Physics, Vol. 85)" pp 335-364, K. Wandelt and S. Thurgate (editors), Springer, New York, 2003.
- Scanning Electrochemical Microscopy, B. R. Horrocks, in "Instrumentation and Electroanalytical Chemistry" pp 444-490, P. R. Unwin (editor), "Encyclopedia of Electrochemistry" A. J. Bard and M. Stratmann (editors), Vol. 3, Wiley-VCH, Weinheim, Germany, 2003.
- Fundamentals of Scanning Electrochemical Microscopy, M. V. Mirkin and B. R. Horrocks, in "Electrochemical Microsystem Technologies (New Trends in Electrochemical Technology, Vol. 2)" pp 66-103, J. W. Schultze, T. Osaka, and M. Datta (editors), Taylor and Francis, New York, 2002.
- Scanning Electrochemical Microscopy, A. J. Bard and M. V. Mirkin (editors), Marcel Dekker, New York, 2001.
- Scanning Electrochemical Microscopy as a Local Probe of Chemical Processes at Liquid Interfaces, A. L. Barker, C. J. Slevin, P. R. Unwin, and J. Zhang, in "Liquid Interfaces in Chemical, Biological, and Pharmaceutical Applications (Surfactant Science Series 95)" pp 283-324, A. G. Volkov (editor), Marcel Dekker, New York, 2001.
- Scanning Electrochemical Microscopy: Beyond the Solid/Liquid Interface, A. L. Barker, M. Gonsalves, J. V. Macpherson, C. J. Slevin, and P. R. Unwin, "Analytica Chimica Acta" Vol. 385, pp 223-240, 1999.
- Scanning Electrochemical Microscopy, A. J. Bard, F.-R. F. Fan, and M. V. Mirkin, in "The Handbook of Surface Imaging and Visualization" pp 667-679, A. T. Hubbard (editor), CRC, Boca Baton, FL 1995.
- Scanning Electrochemical Microscopy, A. J. Bard, F.-R. Fen, and M. Mirkin, in "Physical Electrochemistry: Principles, Methods, and Applications" pp 209-242, I. Rubinstein (editor), Marcel Dekker, New York, 1995.
- Scanning Electrochemical Microscopy, A. J. Bard, F.-R. Fen, and M. Mirkin, in "Electroanalytical Chemistry: a Series of Advances" Vol. 18, pp 244-373, A. J. Bard (editor), Marcel Dekker, New York, 1994.
- Scanning Electrochemical Microscopy. Theory of the Feedback Mode, J. Kwak and A. J. Bard, "Analytical Chemistry" Vol. 61, pp 1221-1227, 1989.
Listings of electrochemistry books, review chapters, proceedings volumes, and full text of some historical publications are also available in the Electrochemistry Science and Technology Information Resource (ESTIR). (http://knowledge.electrochem.org/estir/)
Return to: Top – Encyclopedia Home Page – Table of Contents – Author Index – Subject Index – Search – Dictionary – ESTIR Home Page – ECS Home Page
ECS | Redcat Blog | ECS Digital Library