Return to:
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
ELECTROCHEMICAL BLOOD GLUCOSE TEST STRIPS FOR PEOPLE WITH DIABETES
Ben Feldman
Abbott Diabetes Care
Alameda, CA 94502, USA
(October, 2009)
Diabetes is a condition in which the body either does not produce enough, or does not properly respond to, insulin, a hormone produced in the pancreas that enables cells to absorb glucose in order to turn it into energy. In diabetes, the body either fails to make enough insulin (Type 1 diabetes), or does not properly respond to its own insulin (Type 2 diabetes). This causes glucose to accumulate in the blood, leading to complications of the eyes, kidney, heart and circulatory system, among others. In 2007, the National Diabetes Information Clearinghouse (NDIC) estimated that 23.6 million people, or 7.8% of the population, had diabetes in the United States, of which about 10% was Type 1, with the remainder mostly Type 2.
All people with Type 1 diabetes, and many with Type 2 diabetes, must take additional insulin to control the disease. In order to effectively control Type 1 diabetes, insulin should be injected four or more times daily, including before every meal. This requires frequent self monitoring of the blood glucose concentration, in order to know how much insulin to inject. Typically, before a meal, the patient measures his blood glucose concentration, then calculates the required insulin dosage, based on (i) the measured glucose value, (ii) planned carbohydrate consumption, (iii) known insulin sensitivity, and (iv) a target glucose concentration. If the blood glucose concentration can be effectively controlled, then the long term complications described above can be forestalled. Self monitoring four times daily costs $1,000 � $2,000/year, although most of this expense is typically reimbursed to the patient.
Prior to self monitoring of blood glucose, insulin was usually injected only twice daily by people with Type 1 diabetes, leading to very rudimentary control. The insulin doses were not usually varied, but could be guided over the long term by the measurement of the glucose concentration in urine. Urine glucose measurement is far inferior to blood glucose measurement, since (i) glucose only appears in the urine when it has exceeded a threshold level in blood, which varies from person to person, and (ii) the urine measurement integrates a glucose value over the time required for the bladder to fill.
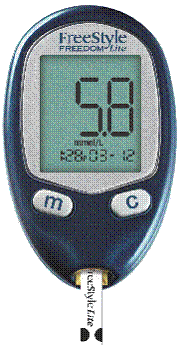 |
| Fig. 1. A handheld blood glucose meter with attached test strip. |
Self monitoring of blood glucose is accomplished using a blood glucose meter and test strips, pictured in Figure 1. Current meters are handheld, with simple intuitive user interfaces that are usually activated automatically by insertion of a test strip. Meters also, to varying extents, have the ability to store, recall, and graph past blood glucose measurements.
The user performs the following sequence of steps in order to obtain a blood glucose measurement:
- Insert a disposable test strip into the meter, automatically turning the meter on.
- Lance the skin with a fine sharp to produce a small blood droplet, about one mm in diameter (volume = 0.3 to 5 µl)
- Sample the blood with the test strip, attached to the meter.
- The meter interrogates the strip in order to measure the blood glucose concentration, typically requiring about 5-10 seconds.
The test strips described in the above procedure can be either optical, in which glucose-derived electrons effect a change in the color of an indicating dye molecule, or electrochemical, in which case the glucose-derived electrons are routed directly through the external meter, and counted. For various reasons, including accuracy and the ability to measure extremely small blood volumes, electrochemical strips have gained the advantage in recent years, and now comprise a growing majority of the test strip market.
Electrochemical blood glucose test strips (EBGTS)
The electrochemical blood glucose test strip (EBGTS) is a very small volume (often about one µl or less) disposable electrochemical cell, which is contacted with whole blood. It then produces, in conjunction with a test meter, an electrical current which is proportional to the blood glucose concentration. Typical currents are a few µA.
This electrical current is produced by the very selective oxidation of glucose in the blood sample, which is catalyzed by two reagents which are precoated inside the test strip: (i) an enzyme, which reacts directly with the glucose molecule to remove its two available electrons, and (ii) a mediator molecule, which takes (either singly or as a pair) the two electrons from the enzyme, and transports them to the working electrode, where they can be measured. This process is illustrated in Figure 2.
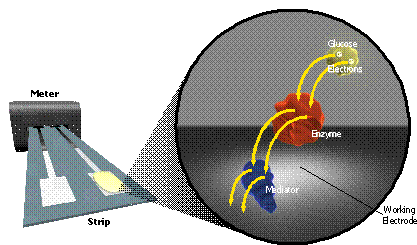 |
 |
| Fig. 2. Electrons flow from glucose to an enzyme to a redox mediator to the working electrode. |
Fig. 3. The first electrochemical blood glucose test strip, ExacTech, launched in 1988. |
The enzyme and mediator act as a sort of electron bucket brigade to transport electrons from glucose to the working electrode. Each enzyme and mediator molecule can repeat this transfer again and again, if necessary. There are a wide variety of different enzymes and mediators which are suitable for use in an EBGTS, this is discussed in more detail below. Although there are many differences between the various commercially available test strips, they all rely on the fundamental mechanism of Figure 2.
This approach was pioneered by Anthony Turner at Cranfield University and Allen Hill at Oxford in 1984. Their work was first incorporated into a commercially available device in 1988, the ExacTech strip shown in Figure 3.
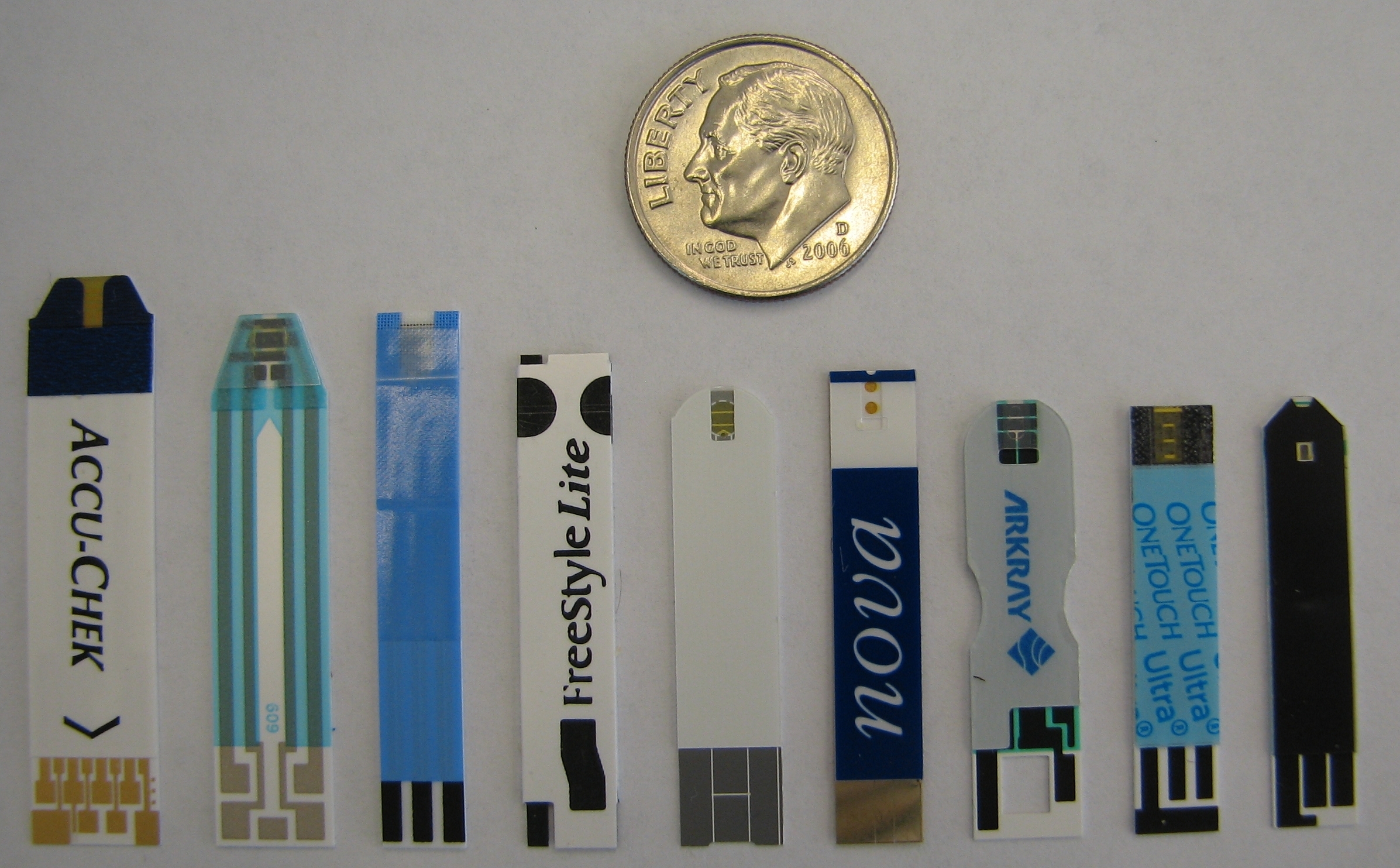 |
| Fig. 4. Some commercially available electrochemical blood glucose test strips. From left: Accuchek Aviva, True Track, Precision Xtra, FreeStyle Lite, Ascensia Contour, Nova, Arkray, One Touch Ultra, Agamatrix. |
The ExacTech strip was constructed from two electrodes, one carbon (the working electrode), and one a mixture of silver and silver chloride (Ag/AgCl, a combination electrode serving both as the counter electrode and as the reference electrode). The working electrode was also coated with a mixture of ferrocene (mediator) and glucose oxidase (enzyme). The electrodes were exposed, unlike current EBGTS, which generally enclose the electrodes in a thin capillary chamber. Therefore the required blood volume (about 10 µl) was much larger than that used by today�s strips. The test time, 30 seconds, was also considerably larger than the 5-10 seconds common today. A series of continuous innovations has led to the much improved performance available today.
Figure 4 shows a representative sample of currently available electrochemical test strips, which vary somewhat in size, but are generally about an inch long and 1/4 inch (about 6 mm) wide. This size is dominated by ergonomic (handling) concerns, since the actual sample chamber (which fills with blood) is only a few mm long, and is located at the top of the strips in Figure 4. The other (bottom) end of the strips contains electrical contacts for communicating with a blood glucose meter.
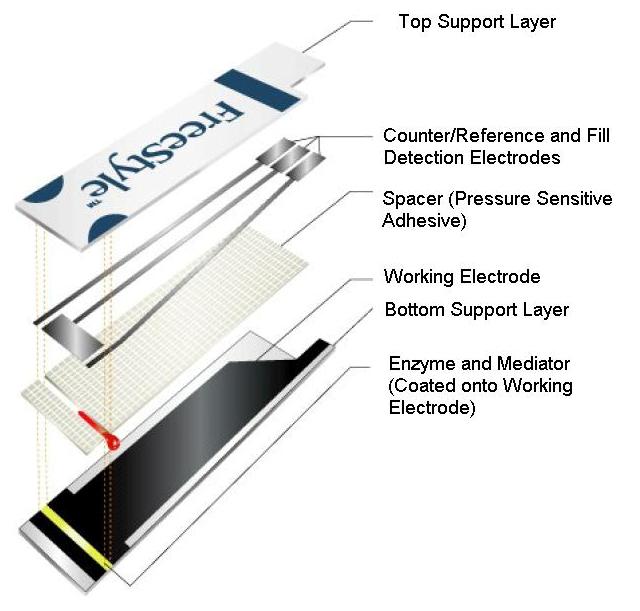 |
| Fig. 5. Diagram of a commercially available blood glucose test strip (Abbott Diabetes Care Inc. FreeStyle® blood glucose test strip) showing fundamental structural components. |
The sample chamber, which is a miniature electrochemical cell, generally is formed from a number of elements and Figure 5 shows these in a cut-away view of an exemplary glucose test strip.
- Plastic supports. Two strips of thin plastic onto which the electrodes and chemical reagents (both described below) can be attached or coated.
- Spacer material. This separates the two plastic supports, creating an internal space, more or less in the shape of a rectangular solid, which can fill with blood. This is typically 50-200 µm thick.
- A working electrode. This is the portal through which electrons from glucose can enter the meter to be measured. It is constructed of an inert material, such as carbon, gold, or platinum.
- The combination �counter/reference� electrode. This is the portal through which glucose derived electrons re-enter the strip after measurement. It is generally constructed from either a mixture of silver and silver chloride, or an inert material similar such as carbon, platinum or gold.
- Chemical reagents. These include principally the enzyme and mediator (described conceptually above in Figure 2), as well as a host of other reagents, including preservatives (to improve shelf life), surfactants (to help blood fill the strip quickly) and film formers (to distribute all of the reagents evenly within the strip), among others.
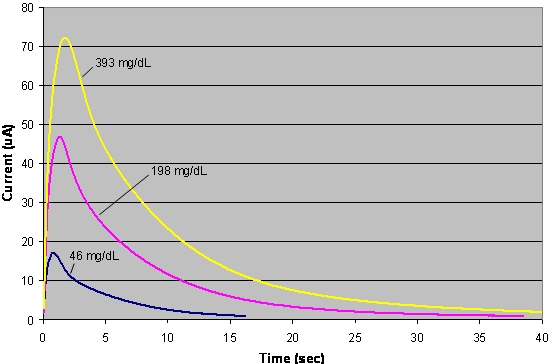 |
| Fig. 6. Representative currents from EBGTS. Currents from high, medium, and low glucose from the FreeStyle test strip of Figure 5. |
When blood is added to an EBGTS, and the proper potential is applied across the electrodes, current flows as glucose is oxidized. Figure 6 shows the current output that occurs when the test strip of Figure 5 is filled with blood, and a potential of +100 mV is applied between the working and �counter/reference� electrodes. The curves rise rapidly, corresponding to the entrance of blood into the strip. In approximately one second, the current peaks, indicating that the strip has filled with blood. The current then decreases, as glucose is consumed, causing its concentration in the vicinity of the working electrode to decrease.
Three different curves are shown, corresponding to low, medium, and high blood glucose levels. The peak currents are proportional to the glucose concentration, although for this particular strip, designed for coulometric analysis, that relationship is non-linear. Instead, the area under the current-time curves varies directly with glucose in a linear fashion.
Methods of electrochemical analysis
All EBGTS produce a time varying current (like that in Figure 6) which must be analyzed to deduce a glucose concentration. However, the method of analysis varies substantially, depending upon the strip design. In the broadest sense, the analysis can be either amperometric, in which the instantaneous current at a particular time is proportional to glucose, or coulometric, in which the integrated charge underneath the entire curve is proportional to glucose.
 Amperometric analysis Amperometric analysis
Amperometric strips predominate among electrochemical test strips. Typically the current is measured 5-15 seconds after the strip is filled with blood, and that current level is directly proportional to the glucose concentration. There are, however, many variations on this theme. For example:
- Current can be measured and averaged over a short (a few seconds) interval.
- Peak current can be measured.
- A mathematical analysis of the declining portion of the current-time curve (termed a Cottrell analysis) can be performed.
In general, strips designed for amperometric analysis share some features:
- The surface area and surface roughness of the working electrode must be highly reproducible to insure that current-time curves are reproducible from strip to strip.
- The electrode surface-area/sample-volume ratio is low, so that glucose is not substantially depleted in the strip during the analysis.
 Coulometric analysis Coulometric analysis
Coulometric strips are less common; they function by exhaustively depleting the glucose in the strip. In these strips, the total charge, or area under the current-time curve is measured, and is proportional to the glucose concentration. Although it may take a considerable amount of time for all the glucose to react, in practice the current-time curve assumes a reproducible exponential shape after a few seconds, and can be easily extrapolated. This leads to an analysis time similar to that for amperometric strips, about 5 seconds for the fastest analyses.
Strips designed for coulometric analysis share some features:
- The internal volume of the sample chamber must be highly reproducible to insure that the analyzed volume is constant from strip to strip. Working electrode roughness is not a dominating factor, as it is in amperometric strips.
- The electrode surface-area/sample-volume ratio is high, so that glucose is completely, or almost completely depleted during the analysis.
Coulometric strips have very robust performance, and are less susceptible to external influences such as variable temperature or hematocrit (red blood cell count), although it is possible to compensate for these effects in amperometric strips.
Calibration of EBGTS
Once a signal, whether charge or current, is extracted from the EBGTS, it must be converted to blood glucose concentration. This is generally done in a process called calibration.
- A series of blood samples are prepared with glucose concentrations spanning the range of interest, typically 200-5000 mg/l.
- The blood samples are tested in a highly accurate laboratory glucose analyzer, called the reference method, to determine the reference glucose value for each sample.
- The samples are then analyzed in EBGTS, to obtain the characteristic signal, which may be current (µA) or charge (µC).
- The signal (current or charge) is plotted against the reference glucose value.
- The slope and intercept of the resulting line are used to assign a calibration code to the EBGTS.
- The user inputs this code into the meter, before self testing, which allows the meter to accurately convert the electrochemical signal to a blood glucose value.
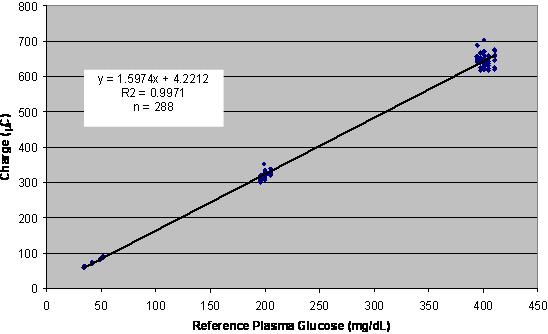 |
| Fig. 7. Calibration curve for a batch of coulometric test strips. |
This process is shown in Figure 7, a plot of charge against reference glucose for a batch of EBGTS. The batches can be quite large, several hundred thousand strips, and a few hundred are typically selected at regular intervals to test.
In the calibration above, the strips are tested with whole blood, while the reference value is obtained from plasma derived from the same whole blood samples. This process, known as plasma calibration, allows glucose test strips to report a plasma glucose concentration, even though they are actually filled with whole blood during use.
Enzymes and mediators
All EBGTS include an enzyme and a mediator, described conceptually above, to ferry electrons from glucose to the working electrode. However, the actual enzymes and mediators used vary substantially from strip to strip. The chemical details of some of the most commonly used ones are described in the Appendix.
Advanced EBGTS features
In addition to the fundamental structural elements described above, many EBGTS are equipped with advanced features to enhance the user experience.
 Fill detection Fill detection
Most EBGTS are equipped with some means of determining that the sample chamber is entirely filled with blood. This is important, because a partially filled chamber can lead to erroneous, generally low, results. Older strips relied on visual confirmation of fill, but that process has been automated in newer strips. There are three basic ways to accomplish this:
- The combination �counter/reference� electrode is placed �downstream� of the working electrode in the sample chamber. Therefore, as the electrochemical circuit is completed by blood contacting the counter/reference electrode, the working electrode is guaranteed to be completely covered by blood.
- Dual working electrodes (one downstream from the other) are used. Their outputs are compared, and must correlate closely in order for the strip to be deemed full.
- Extra electrodes (beside working and counter/reference) are included, whose function is simply to sense the fluid level. An example is shown in Figure 5.
 Automatic coding Automatic coding
Generally, calibration codes, corresponding to a particular batch of strips, are entered into the meter by the user, either manually or through insertion of a calibration �chip,� that accompanies the strip vial. However, it is desirable to entirely bypass this process, since user error is always a possibility. Therefore, some EBGTS do not require calibration of any sort. This is generally accomplished by:
- Precise manufacturing control of the sensitivity of the produced strips, such that their performance is highly reproducible from batch to batch. In this process, strips are rejected, and disposed of, if their performance does not meet very stringent performance criteria.
- Adding an indicating mark to the strip that the meter can use to assign a calibration code.
Both systems are currently in use, and automatically coded strips make up an increasing share of EBGTS.
 Hematocrit correction Hematocrit correction
Hematocrit is the percentage, by volume, of red blood cells, in a blood sample. The higher the hematocrit, the thicker the blood. Hematocrit affects blood glucose measurements by (i) changing the quantity of oxygen dissolved in the blood sample, which can affect strips that use glucose oxidase, and (ii) changing the viscosity of the sample, which affects the rate at which glucose mediator and enzyme can diffuse and react.
Historically, measures have been taken during strip design to minimize the hematocrit effect, for example, excluding red blood cells from near the working electrode surface. An alternate approach is to measure the hematocrit, and correct the reported blood glucose value. For example, hematocrit can be measured by using an extra pair of electrodes to measure the conductivity of the blood sample, by applying an ac signal between the electrodes. Conductivity is a strong function of hematocrit, so this is an effective form of correction. As regulatory standards become more stringent, this sort of correction is likely to increase.
Appendix
 Enzymes Enzymes
 |
| Table I. Commonly used enzymes for EBGTS |
 |
| Enzyme |  Reacts with O2? Reacts with O2?  |  Reacts with maltose? Reacts with maltose?  |
 |
 Glucose Oxidase Glucose Oxidase | Yes | No |
 PQQ-Glucose Dehydrogenase PQQ-Glucose Dehydrogenase | No | Yes |
 NAD-Glucose Dehydrogenase NAD-Glucose Dehydrogenase | No | No |
 FAD-Glucose Dehydrogenase FAD-Glucose Dehydrogenase | No | No |
 |
Four enzymes are commonly used in these strips, and these are listed in Table I.
Glucose oxidase was first employed in EBGTS, and is still used in a few commercially available test strips, although its use is declining, since it reacts rapidly with oxygen. EBGTS made with glucose oxidase produce currents that are dependent on the amount of dissolved oxygen in the blood sample, which is heavily dependent on the hematocrit (% red blood cells) in the sample.
Glucose oxidase has been largely supplanted by the dehydrogenases, which do not react appreciably with oxygen. (In the table above: PQQ, NAD, and FAD refer to the electron transferring cofactors, which differ between each of the distinct enzymes.) However PQQ-GDH does react with many non-glucose sugars, particularly maltose, which can be elevated in patients undergoing peritoneal dialysis. For this reason, use of PQQ-GDH is decreasing, and the FDA has recently restricted its use in new commercial products.
Both NAD-GDH and FAD-GDH combine oxygen rejection with high specificity for glucose. Their use in EBGTS is increasing. For all the enzymes described, the amount used per strip is typically 1-10 enzymatic units, where a unit represents the enzymatic activity capable of oxidizing one micromole of glucose/minute, at 37oC (99oF). Enzymes typically are deposited on at least the working electrode, and sometimes on one entire side of the sample chamber, and are codeposited with buffers and stabilizing agents, such as bovine serum albumin.
 Mediators Mediators
A wide variety of mediator is used in EBGTS, but they share certain common features:
- Rapid reactivity with both the enzyme and the working electrode material of the particular strip in question.
- High solubility in blood.
- Capable of being easily stabilized in the oxidized state; this reduces spurious non-glucose related background currents.
A few commonly used mediators are: (i) ferricyanide, (ii) 1,10-phenanthroline quinone, and (iii) osmium-based mediators. Their oxidation potentials are 500, 100, and -120 mV (against Ag/AgCl reference), respectively. But this list is by no means exhaustive. Their chemical structures are shown below.
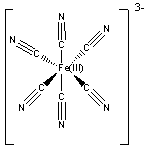 |
| Ferricyanide. |
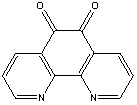 |
| 1,10-phenanthroline quinone. |
 |
| An osmium-based mediator. |
In general, as the potential of the mediator increases, the strip becomes more liable to spurious currents from electrochemical interferents such as ascorbate, urate, and acetaminophen. In that sense, low potential mediators are more desirable, although alternate strategies (such as a second working electrode with no deposited enzyme) can be used to quantify, then correct for interferent currents.
Related articles
Electrochemical nose
Electrochemical sensors
Jaroslav Heyrovsky and polarography
The past, present, and future of electroanalytical chemistry
Bibliography
- Electrochemical Glucose Sensors and Their Applications in Diabetes Management, Adam Heller and Ben Feldman �Chemical Reviews� Vol. 108, pp 2482-2505, 2008.
- The Technology Behind Glucose Meters: Test Strips, Joachim Hones, Peter Muller, and Nigel Surridge, �Diabetes Technology and Therapeutics� Vol. 10, No. S1, pp S10-S26, 2008.
- Recent Progress in Analytical Instrumentation for Glycemic Control in Diabetic and Critically Ill Patients, Venkata R. Kondepati and H. Michael Heise, �Analytical and Bioanalytical Chemistry� Vol. 338, pp 545-563, 2007.
- Commercial Biosensors: Applications to Clinical, Bioprocess, and Environmental Samples, T. P. Henning and D. D. Cunningham, John Wiley and Sons, New York, 1998.
- Ferrocene-Mediated Enzyme Electrode for Amperometric Determination of Glucose, Anthony E. G. Cass, Graham Davis, Graeme D. Francis, H. Allen O. Hill, William J. Aston, I. John Higgins, Elliot V. Plotkin, Lesley D. L. Scott, and Anthony P. F. Turner, �Analytical Chemistry� Vol. 56, pp 667-671, 1984.
Listings of electrochemistry books, review chapters, proceedings volumes, and full text of some historical publications are also available in the Electrochemistry Science and Technology Information Resource (ESTIR). (http://knowledge.electrochem.org/estir/)
Return to:
Top –
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
|




 Mediators
Mediators 









