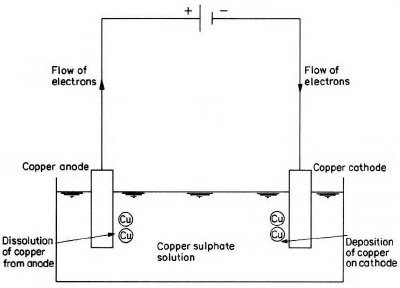
A cation reaching the cathode is neutralized, or discharged, by the negative electrons on the cathode. Since the cation is usually the positively charged atom of a metal, the result of this reaction is the deposition of metal atoms. To maintain the cathodic reaction, electrons are required to pass around the external circuit. These are obtained from the atoms of the metal anode, and these atoms thus become the positively charged cations which pass into solution. In this case, the reaction is the reverse of the cathodic reaction.
The electrolyte in its bulk must be electrically neutral; that is, there must be equal numbers of opposite charges within it, and thus there must be equal amounts of reaction at both electrodes. Therefore, in the electrolysis of copper sulphate solution with copper electrodes, the overall cell reaction is simply the transfer of copper metal from the anode to the cathode. When the wires are weighted at the end of the experiment, the anodic wire will be found to have lost weight, whilst the cathodic wire will have increased in weight by an amount equal to that lost by the other wire. Some examples of the reactions occurring in these processes are shown in the Appendix.
These results are embodied in Faraday�s two laws of electrolysis:
A popular application of electrolysis is the electroplating process in which metal coatings are deposited upon the surface of a cathodically polarized metal. An example of an anodic dissolution operation is electropolishing. Here, the item which is to be polished is made the anode in an electrolytic cell. Irregularities on its surface are dissolved preferentially so that, on their removal, the surface becomes flat and polished. ECM is similar to electropolishing in that it also is an anodic dissolution process. But the rates of metal removal offered by the polishing process are considerably less than those needed in metal machining practice. Some observations relevant to ECM can be made:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
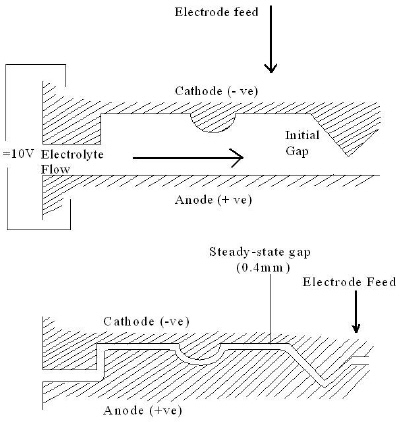 |
| Fig. 3. Working principles of ECM. |
![]() •
• ![]() the rate of metal machining does not depend on the hardness of the material,
the rate of metal machining does not depend on the hardness of the material,
![]() •
• ![]() complicated shapes can be machined on hard metals,
complicated shapes can be machined on hard metals,
![]() •
• ![]() there is no tool wear.
there is no tool wear.
The schematic of an industrial �electrochemical machine� is shown in Figure 4, and an actual example of a cathode tool and anode workpiece are shown in Figure 5.
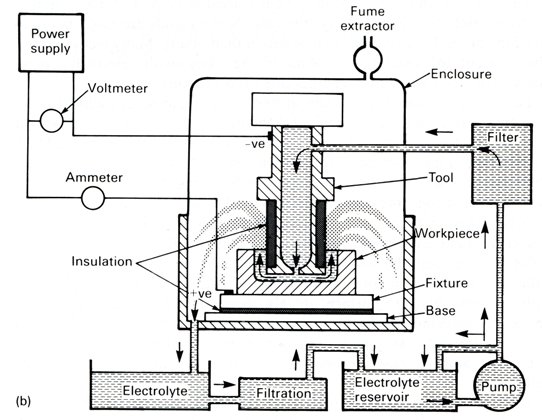 |
| Fig. 4. Industrial electrochemical machine. |
 |
| Fig. 5. Example of cathode tool (above) and anode workpiece (below). |
Electrochemical machining
 Machine components
Machine components
Industrial electrochemical machines work on the principles outlined. Particular attention has to be paid to the stability of the electrochemical machine tool frame, and to the machining table which should also be stable and firm. The electrolyte has to be filtered carefully to remove the products of machining and often has to be heated in its reservoir to a fixed temperature, for instance 30oC (86oF), before entering the machining apparatus. This procedure is used to provide constant operating conditions. During machining the electrolyte heats up from the passage of current. Precautions must be taken to avoid a high electrolyte temperature which can cause changes in the electrolyte specific conductivity and subsequent undesirable effects on machining accuracy.
 Rates of machining
Rates of machining
The rates at which metals can be electrochemically machined is in proportion to the current passed through the electrolyte and the elapsed time for that operation, and is in inverse proportion to the electrochemical equivalent of the anode-metal which corresponds to the atomic weight of the dissolving ions over the ionic charge times the Faraday�s constant. See the Appendix for more details.
Many factors other than current influence the rate of machining. These involve electrolyte type, rate of electrolyte flow, and some other process conditions. For example current efficiency decreases when current density is increased for a certain metal, for example, for nickel.
If the ECM of titanium is attempted in sodium chloride electrolyte, usually very low (10�20%) current efficiencies are obtained. When this solution is replaced by some mixture of fluoride-based electrolytes, to achieve greater efficiencies (>60%), a higher voltage is used.
If the rates of the flow are kept too low, the current efficiency of even the most easily electrochemically machined metal is reduced. Insufficient flow does not allow the products of machining to be so readily flushed from the machining gap. When complex shapes have to be produced the design of tooling incorporating the right kind of flow ports becomes a considerable problem.
 Surface finish
Surface finish
Type of electrolytes used in the process affects the quality of surface finish obtained in ECM. Depending on the material, some electrolytes leave an etched finish. This finish results from the nonspecular reflection of light from crystal faces electrochemically dissolved at different rates. Sodium chloride electrolyte tends to produce an etched, matte finish with steels and nickel alloys.
The production of an electrochemically-polished surface is usually associated with the random removal of atoms from the anode workpiece, whose surface has become covered with an oxide film. This is governed by the metal-electrolyte combination used. Nonetheless, the mechanisms controlling high-current density electropolishing in ECM are still not completely understood. For example, with nickel-based alloys, the formation of a nickel oxide film seems to be a prerequisite for obtaining a polished surface; a finish of this quality, of 0.2 µm, has been claimed for Nimonic (a nickel alloy) machined in saturated sodium chloride solution. Surface finishes as fine as 0.1 µm have been reported when nickel-chromium steels are machined in sodium chlorate solution. The formation of an oxide film on the metal surface is considered the key to these conditions of polishing.
Sometimes the formation of oxide film on the metal surface hinders efficient ECM and leads to poor surface finish. For example, the ECM of titanium is rendered difficult in chloride and nitrate electrolytes because the oxide film formed is so passive. Even when higher voltages about 50 V are applied to break the oxide film, its disruption is so non-uniform that deep grain boundary attack of the metal surface can occur.
Occasionally, metals that have undergone ECM have a pitted surface while the remaining area is polished or matte. Pitting normally stems from gas evolution at the anode; the gas bubbles rupture the oxide film.
Process variables also affect surface finish. For example, as the current density is raised the finish generally becomes smoother on the workpiece surface. A similar effect is achieved when the electrolyte velocity is increased. In tests with nickel machined in hydrochloric acid solution the surface finish has been noted to improve from an etched to a polished appearance when the current density is increased from about 8 to 19 A/square centimeter with constant flow velocity.
 Accuracy and dimensional control
Accuracy and dimensional control
Electrolyte selection plays an important role in ECM. Sodium chloride, for example, yields much less accurate components than sodium nitrate. The latter electrolyte has far better dimensional control owing to its current efficiency - current density characteristics. Using sodium nitrate electrolyte, the current efficiency is greatest at the highest current densities. In hole drilling these high current densities occur between the leading edge of the drilling tool and the workpiece. In the side gap there is no direct movement between the tool and workpiece surface, so the gap widens and the current densities are lower. The current efficiencies are consequently lower in the side gap and much less metal than predicted from Faraday�s law is removed. Thus the overcut in the side gap is reduced with this type of electrolyte. If another electrolyte such as sodium chloride solution was used instead, then the overcut could be much greater. Using sodium chloride solutions, its current efficiency remains steady at almost 100% for a wide range of current densities. Thus, even in the side gap, metal removal proceeds at a rate which is mainly determined by current density, in accordance with Faraday�s law. A wider overcut then ensues.
 Shaping
Shaping
Most metal-shaping operations in ECM utilize the same inherent feature of the process whereby one electrode, generally the cathode tool, is driven toward the other at a constant rate when a fixed voltage is applied between them. Under these conditions, the gap width between the tool and the workpiece becomes constant. The rate of forward movement between the tool and the workpiece becomes constant. The rate of forward movement of the tool is matched by the rate of recession of the workpiece surface resulting from electrochemical dissolution.
Three practical cases are of interest in considering some equations derived for the variation of the interelectrode gap width:
- When there is no tool movement, the gap width increases indefinitely with the square root of machining time. This condition is often used in deburring by ECM when surface irregularities are removed from components in a few seconds, without the need for mechanical movement of the electrode.
- When the tool is moved mechanically at a fixed rate toward the workpiece, the gap width tends to a steady value. This inherent feature of ECM, whereby an equilibrium gap width is obtained, is used widely in ECM for reproducing the shape of the cathode tool on the workpiece.
- Under short-circuit conditions the gap width goes to zero. If some process conditions, such as too small equilibrium gap width caused by a too high movement of the tool toward the workpiece, occur, contact between the two electrodes ensues. This causes a short circuit between the electrodes and hence premature termination of machining.
The equilibrium gap is applied widely in the shaping process. Studies of ECM shaping are usually concerned with three distinct problems:
- The design of the cathode tool shape needed to produce a required profile geometry of the anode workpiece.
- For a given cathode tool shape, prediction of the resultant anode workpiece geometry, for example, hole drilling by ECM.
- Specification of the shape of the anode workpiece, as machining proceeds. This is most readily predicted for the smoothing of surfaces, although for actual shaping of components by ECM, estimates of the machining times as the shape develops provide useful information about the process.
Applications
 Smoothing of rough surfaces
Smoothing of rough surfaces
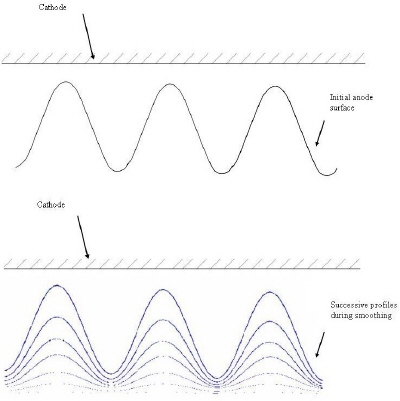 |
| Fig. 6. Smoothing of rough surfaces. |
Electrochemical deburring is a fast process; typical times for smoothing the surfaces of manufactured components are 5 to 30 seconds. Owing to its speed and simplicity of operation, electrochemical deburring can be performed with a fixed, stationary cathode tool. The process is used in many industries.
 Hole drilling
Hole drilling
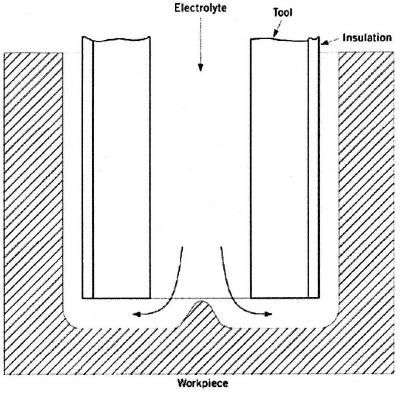 |
| Fig. 7. Electrochemical hole drilling. |
The main machining action is carried out in the gap formed between the leading edge of the drill tool and the base of the hole in the workpiece. ECM also proceeds laterally between the side walls of the tool and component, where the current density is lower than at the leading edge of the advancing tool. Since the lateral gap width becomes progressively larger than that at the leading edge, the side-ECM rate is lower. The overall effect of the side-ECM is to increase the diameter of the hole produced. The distance between the side wall of the workpiece and the central axis of the cathode tool is larger than the external radius of the cathode. This difference is known as the "overcut". The amount of overcut can be reduced by several methods. A common procedure involves the insulation of the external walls of the tool (Figure 7), which inhibits side-current flow. Another practice lies in the choice of an electrolyte such as sodium nitrate, which has the greatest current efficiency at the highest current densities. In hole drilling these high current densities occur between the leading edge of the drill and the base of the workpiece. If another electrolyte such as sodium chloride were used the overcut could be much greater. The current efficiency for sodium chloride remains steady at almost 100% for a wide range of current densities. Thus, even in the side gap, metal removal proceeds at a rate that is mainly determined by the current density, in accordance with Faraday's law.
Holes with diameters of 0.05 to 75 millimeter have been achieved with ECM. For holes of 0.5 to 1.0 millimeter diameter, depths of up to 110 millimeter have been produced. Drilling by ECM is not restricted to round holes; the shape of the workpiece is determined by that of the tool electrode.
 Full-form shaping
Full-form shaping
Full-form shaping utilizes a constant gap across the entire workpiece and the tool is moved mechanically at a fixed rate toward the workpiece in order to produce the type of shape used for the production of compressor and turbine blades. In this procedure, current densities as high as 100 A/square centimeter are used, and across the entire face of the workpiece, the current density remains high.
Electrolyte flow plays an even more influential role in full-form shaping than in drilling and smoothing of surfaces. The entire large cross-sectional area of the workpiece has to be supplied by the electrolyte as it flows between electrodes. The larger areas of electrodes involved mean that comparatively higher pumping pressures and volumetric flow rates are needed.
 Electrochemical grinding
Electrochemical grinding
The main feature of electrochemical grinding (ECG) is the use of a grinding wheel in which an insulating abrasive, such as diamond particles, is set in a conducting material. This wheel becomes the cathode tool. The nonconducting particles act as a spacer between the wheel and workpiece, providing a constant interelectrode gap, through which electrolyte is flushed.
Accuracies achieved by ECG are usually about 0.125 millimeter. A drawback of ECG is the loss of accuracy when inside corners are ground. Because of the electric field effects, radii better than 0.25 � 0.375 millimeter can seldom be achieved.
A wide application of electrochemical grinding is the production of tungsten carbide cutting tools. ECG is also useful in the grinding of fragile parts such as hypodermic needles.
 Electrochemical arc machining
Electrochemical arc machining
A process that relies on electrical discharges in electrolytes, thereby permitting metal erosion as well as ECM in that medium, has been developed. Because this process relies on the onset of arcs rather than sparks, it has been named electrochemical arc machining (ECAM). A spark has been defined as a sudden transient and noisy discharge between two electrodes; an arc is a stable thermionic phenomenon. Duration discharges of approximately 1 second to 1 millisecond are described as sparks, whereas for durations of about 0.1 second said discharges can be considered arcs. Because in the ECAM process duration, energy, and time of ignition of sparks are under control, it is valid to regard them as arcs.
An attraction of the ECAM technique is the very fast rates of metal removal attainable by the combined effects of sparking and ECM. The ECAM technique can be applied in all the ways discussed for ECM, thus surfaces can be smoothed and drilled. Turning is also possible, as is wire machining.
One form of this process relies on a pulsed direct current, that is, full-wave rectified ac power supply that is locked in phase with a vibrating tool head. The oscillation of the tool gives rise to a set of conditions whereby ECM occurs over each wave cycle. The interelectrode gap narrows as the tool vibrates over one cycle. During the same period the current rises until sparking takes place by breakdown of the electrolyte and/or generation of electrolytic gas or steam bubbles in the gap, the production of which aids the discharge process.
For drilling, the discharge action occurs at the leading edge of the tool, whereas ECM takes place on the side walls between the tool and the workpiece. The combined spark erosion and ECM action yields fast rates of metal removal. Because ECM is still possible, any metallurgical damage to the components caused by the sparking action can be removed by a short period of ECM after the main ECAM action. Currents of 250 A at 30 V are typically used in the process.
Economic aspects
The industrial sectors utilizing ECM technology fall into five main categories: tool and die, automotive, aerospace, power generation, and oil and gas industries. Leading the world�s principle machine tool manufacturing nations in production and export of tools in the 1980s were Japan followed by the former West Germany. The United States led in imports and consumption; consumption was high for both Japan and W. Germany as well. Unconventional machine tools including ECM are generally considered to account for only 1% of total production. Electrodischarge machining (EDM) holds the largest share, possibly as much as 50% and ECM about 15% lagging behind laser processes which are 20%.
Manufacturing engineers wishing to use ECM processes in industry need to address the challenge of proper tool design. The cost of design can be as much as 20% of the cost of an electrochemical machine for complex components. Predictability of overcuts obtained for specific applications and the particular electrolytes to be used for the alloy metals that have to be machined must also be considered along with specific controls and limits on the ECM equipment needed.
Computer-controlled equipment and sensors are available for electrochemical machining systems. However in the 1990s practical ECM systems are often favored because the amount of control and/or monitoring of the process is far less than that which was required in the 1970s. Thus machines are used successfully in which electrical spark detection is eliminated and machining products control, for example, pH monitoring, is nonexistent.
The present and future status of ECM
High-rate anodic electrochemical dissolution is a practical method of smoothing and shaping hard metals by employment of simple aqueous electrolyte solutions without wear of the cathodic tool. ECM can offer substantial advantages in a wide range of cavity-sinking and shaped-hole production operations.
Control of the ECM process is improving all the time, with more sophisticated servo-systems, and better insulating coatings. However there is still a need for basic information on electrode phenomena at both high current densities and electrolyte flow-rates.
Tool design continues to be of paramount importance in any ECM operation. The use of computer-aided design to predict cathode tool profiles will continue to advance.
Recently developments in ECM practice have dwelt on the replacement of constant dc by pulsed currents (PECM). Significant improvements in surface quality have been claimed. Much smaller electrode gaps may be obtained, for example, below 0.1 millimeter leading to improved control of accuracy, for example to 0.02 to 0.10 millimeter, with dies, turbine blades, and precision electronic components. The key to further advancement in PECM lies in development of a low cost power supply. Successful development of technique will enable on-line monitoring of the gap size, enabling closer process control.
Despite these attractions, PECM should be regarded as complementary to, and not a substitute for, established ECM technology; the former is expensive and metal removal rates can be lower than these of the latter.
The advent of new technology for controlling the ECM process and the development of new and improved metal alloys, which are difficult to machine by conventional means, will assure the future of electrochemical machining.
 Electrolysis
Electrolysis
Reactions that occur during the electrolysis of copper sulphate (Figure 1) are as follows. The anodic reaction is ionizing of copper:
Cu ==> Cu2+(aq) + 2e-
While at the cathode the copper ions are discharged to form copper metal:
Cu2+(aq) + 2e- ==> Cu
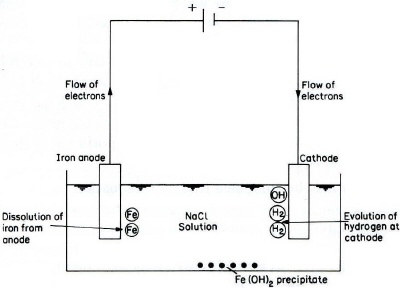
Reactions that occur during the electrolysis of iron (Figure 2) are as follows. The anodic reaction is ionizing of iron:
Fe ==> Fe2+(aq) + 2e-
At the cathode, the reaction is likely to be the generation of hydrogen gas and the production of hydroxyl ions:
H2O + 2e- ==> H2 + 2OH-
The net reaction is thus:
Fe + 2H2O ==> Fe(OH)2(s) + H2
The ferrous hydroxide may react to form ferric hydroxide:
4Fe(OH)2 + 2H2O + O2 ==> 4Fe(OH)3
 Characteristics of ECM
Characteristics of ECM
By use of Faraday�s laws, if �md� (kg) is the mass of metal dissolved, and because �md = vd� where �v� (m3) is the corresponding volume and �d� (kg/m3) the density of the anode metal, the volumetric removal rate of anode metal (m3/second) is given by:
![]()
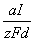
Where �a� (kg/mol) is the atomic weight of the anode metal, �I� (ampere) is the current flowing, �z� is the ionic charge of the anode metal, and the Faraday constant �F� equals 96,487 coulombs/mol. If a machining operation has to be carried out on an iron workpiece at a typical rate of 2.6 × 10-8 kg/C, for this removal rate to be achieved by ECM, the current in the cell must be about 700 A, because �a/zF� = 29 × 10-8 and �d�= 7,860 kg/m3 for iron.
 Rates of machining
Rates of machining
By use of Faraday�s laws the rates at which metals can be electrochemically machined can be calculated.
![]()
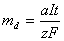
Where �md� (kg) is the mass of metal electrochemically machined by current �I� (ampere) passed for a time �t� (second). The quantity �a/zF� is called the electrochemical equivalent of the anode metal as mentioned before.
Table I shows the metal machining rates that can be obtained when a current of 1000 A is used in ECM. Metal removal rates in terms of volumetric machining are often more useful than mass removal rates, and both quantities are included. (It is assumed that the anodic current efficiency is 100%, that is all the current is used to remove metal, which is not always the case.)
 | |||||
| Table I. Metal machining rates | |||||
 | |||||
| Metal | Atomic | Ionic | Density | Removal rate | |
| weight | charge | 103 kg/m3 | 10-3 kg/s | 10-6 m3/s | |
 | |||||
| Aluminum | 26.97 | 3 | 2.67 | 0.093 | 0.035 |
| Beryllium | 9.0 | 2 | 1.85 | 0.047 | 0.025 |
| Chromium | 51.99 | 2 | 7.19 | 0.269 | 0.038 |
| 3 | 0.180 | 0.025 | |||
| 6 | 0.090 | 0.013 | |||
| Cobalt | 58.93 | 2 | 8.85 | 0.306 | 0.035 |
| 3 | 0.204 | 0.023 | |||
| Niobium | 92.91 | 3 | 9.57 | 0.321 | 0.034 |
| (Columbium) | 4 | 0.241 | 0.025 | ||
| 5 | 0.193 | 0.020 | |||
| Copper | 63.57 | 1 | 8.96 | 0.660 | 0.074 |
| 2 | 0.329 | 0.037 | |||
| Iron | 55.85 | 2 | 7.86 | 0.289 | 0.037 |
| 3 | 0.193 | 0.025 | |||
| Magnesium | 24.31 | 2 | 1.74 | 0.126 | 0.072 |
| Manganese | 54.94 | 2 | 7.43 | 0.285 | 0.038 |
| 4 | 0.142 | 0.019 | |||
| 6 | 0.095 | 0.013 | |||
| 7 | 0.081 | 0.011 | |||
| Molybdenum | 95.94 | 3 | 10.22 | 0.331 | 0.032 |
| 4 | 0.248 | 0.024 | |||
| 6 | 0.166 | 0.016 | |||
| Nickel | 58.71 | 2 | 8.90 | 0.304 | 0.034 |
| 3 | 0.203 | 0.023 | |||
| Silicon | 28.09 | 4 | 2.33 | 0.073 | 0.031 |
| Tin | 118.69 | 2 | 7.30 | 0.615 | 0.084 |
| 4 | 0.307 | 0.042 | |||
| Titanium | 47.9 | 3 | 4.51 | 0.165 | 0.037 |
| 4 | 0.124 | 0.028 | |||
| Tungsten | 183.85 | 6 | 19.3 | 0.317 | 0.016 |
| 8 | 0.238 | 0.012 | |||
| Uranium | 238.03 | 4 | 19.1 | 0.618 | 0.032 |
| 6 | 0.412 | 0.022 | |||
| Zinc | 65.37 | 2 | 7.13 | 0.339 | 0.048 |
 | |||||
Bibliography
- Electrochemical machining, J. A. McGeough, in �Kirk-Othmer Encyclopedia of Chemical Technology� (5th edition), Vol. 9, pp 590-606, J. I. Kroschwitz (editor), Wiley-Interscience, NY 2005.
- Machining methods: electrochemical, J. A. McGeough and X. K. Chen, in �Kirk-Othmer Encyclopedia of Chemical Technology� (4th edition), Vol. 15, pp 608-622, J. I. Kroschwitz and M. Howe-Grant (editors), Wiley-Interscience, NY 1995.
- A Study of Electrical Discharges in Electrolyte by High-Speed Photography, X. Ni, J. A. McGeough, and C. A. Greated, �Journal of Electrochemical Society� Vol. 140, pp 3505-3512, 1993.
- Study of Pulse Electrochemical Machining Characteristics, K. P. Rajurkar, J. Kozak, and B. Wei, �Annals International College for Production Research� Vol. 42, pp 231-234, 1993.
- Jet and Laser-Jet Electrochemical Micromachining of Nickel and Steel, M. Datta, L. T. Romankiw, D. R. Vigliotti, and R. J. Von Gutfeld, �Journal of Electrochemical Society� Vol. 136, pp 2251-2256, 1989.
- Advanced Methods of Machining, J. A. McGeough, Chapman and Hall, London, 1988.
- Analysis of Electrochemical Arc Machining by Stochastic and Experimental Methods, A. B. M. Khayry and J. A. McGeough, �Proceedings of the Royal Society of London� Vol. A412, pp 403-429, 1987.
- An Electrochemical Machining Method for Removal of Samples and Defective Zones in Metal Pipes, Vessels and Structures, D. Clifton, J. W. Midgley, and J. A. McGeough, �Proceedings of the Institution of Mechanical Engineers, Part B, Journal of Engineering Manufacture� Vol 201, pp 229-231, 1987.
- Surface Effects on Alloys Drilled by Electrochemical Arc Machining, A. DeSilva and J. A. McGeough, �Proceedings of the Institution of Mechanical Engineers, Part B, Journal of Engineering Manufacture� Vol. 200, pp 237-246, 1986.
- Analysis of Taper Produced on Side Zone During ECD, V. K. Jain and V. N. Nanda, �Precision Engineering, Journal of the American Society for Precision Engineering� Vol. 8, No. 1, pp 27-33, 1986.
- Electrochemical Wirecutting, S. R. Ghabrail and C. F. Noble, in �Proceedings of the 24th International Machine Tool Design and Research Conference� pp 323-328, B. J. Davies (editor), Macmillan, Manchester, UK 1984.
- Drilling Without Drills, G. Bellows and J. D. Kohls, �American Machinist� pp 178-183, 1982.
- Deburring-2: Electrochemical Machining, D. Graham, �The Production Engineering� Vol. 61, No. 6, pp 27-30, 1982.
- Comparative Studies of ECM, EDM and ECAM, I. M. Crichton, J. A. McGeough, W. Munro, and C. White, �Precision Engineering� Vol. 3, pp 155-160, 1981.
- Aspects of Drilling by Electrochemical Arc Machining, T. Drake and J. A. McGeough, in �Proceedings of the 21th Machine and Tool Design and Research Conference� pp 362-369, J. M. Alexander (editor), Macmillan, New York, 1981.
- Basic Study of ECDM-II, M. Kubota, Y. Tamura, H. Takahahi, and T. Sugaya, �Journal Association Electro-Machining� Vol. 13, No. 26, pp 42-57, 1980.
- Basic Study of ECDM-I, M. Kubota, Y. Tamura, J. Omori, and Y. Hirano, �Journal Association Electro-Machining� Vol. 12, No. 23, pp 24-33, 1978.
- Newcomers for Production, G. Bellows, in �Non-Traditional Machining Guide 26� pp 28-29, Metcut Research Associates Inc., Cincinnati, Ohio, 1976.
- Electrochemical machining, J. Kaczmarek, in �Principles of Machining by Cutting, Abrasion and Erosion� pp 487-513, Peregrinus, Stevenage UK, 1976.
- Principles of Electrochemical Machining, J. A. McGeough, Chapman and Hall, London, 1974.
Listings of electrochemistry books, review chapters, proceedings volumes, and full text of some historical publications are also available in the Electrochemistry Science and Technology Information Resource (ESTIR). (http://knowledge.electrochem.org/estir/)
Return to: Top – Encyclopedia Home Page – Table of Contents – Author Index – Subject Index – Search – Dictionary – ESTIR Home Page – ECS Home Page
ECS | Redcat Blog | ECS Digital Library