Most protonophoric uncouplers widely used in photosynthesis research, are oxidized by the manganese cluster of the Photosystem II oxygen-evolving complex in chloroplasts and inhibit photosynthetic water oxidation. Oxidized uncouplers can be reduced by the membrane pool of plastoquinone, leading to formation of an artificial cyclic electron transfer chain around Photosystem II involving uncouplers as redox carriers. Protonophores inhibit the Hill reaction in chloroplast and cyanobacterial membranes. Inhibition of the Hill reaction by uncouplers reaches maximum when the pH corresponds to the pK values of these compounds.
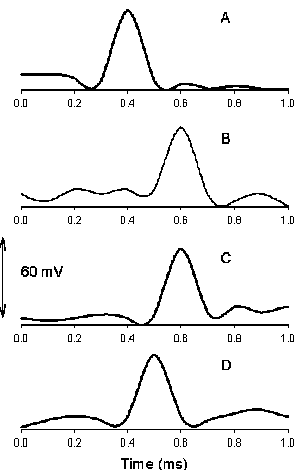
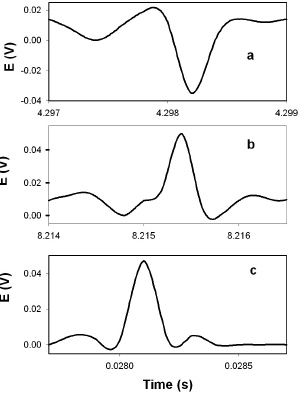
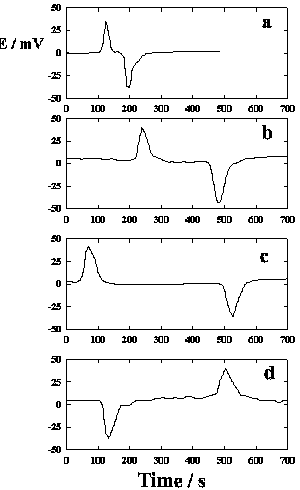
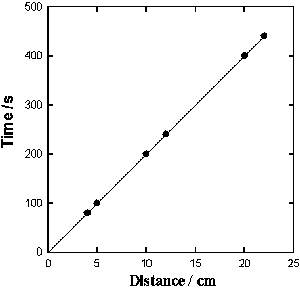
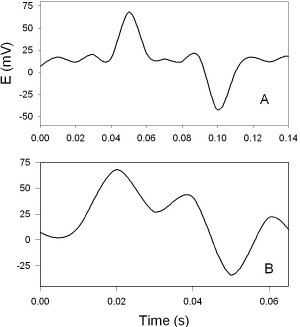
DNP induces fast action potentials and decreases the variation potential to zero in soybeans. The addition of aqueous DNP to the soil induces fast action potentials in soybeans. After treatment of soil by an aqueous solution of DNP, variation potential, measured between two silver/silver chloride microelectrodes in a stem of soybean, slowly decreases from 80-90 mV (negative in a root, positive on the top of the soybean) to zero during a 48-hour time frame. The duration of single action potentials, 24 hours after treatment by DNP, varies from 3 sec to 0.02 sec (Figure 5). The amplitude of action potentials is about 60 mV. The maximum speed of action potential propagation is one m/sec. After two days, the variation potential stabilized at zero. Fast action potentials were generated in a soybean, with amplitude of about 60 mV, 0.02 sec duration time, and a speed of 2 m/sec, after treatment. Fromm and Spanswick studied the inhibiting effects of DNP on the excitability of willow by recording the resting potential in the phloem cells. In willow, 10-4 M DNP rapidly depolarized the membrane potential by about 50 mV.
|
 |
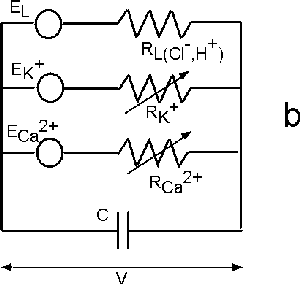 |
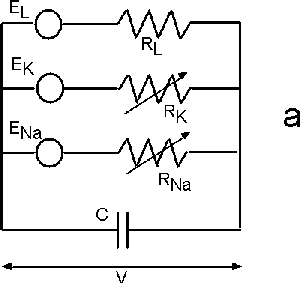
| Fig. 6. The Hodgkin-Huxley (HH) equivalent circuit for an axon (a) and the modified HH circuit for sieve tubes in phloem (b). |
Hodgkin and Huxley formulated a membrane model that accounts for potassium, sodium, and ion leakage channels in squid giant axons (Figure 6a). The membrane resting potential for each ion species is treated like a battery. A variable resistor models the degree to which the channel is open.
Fromm and Spanswick found that electric stimulation of the plant is followed by ion shifts that are most striking in the phloem cells. While the content of potassium and chloride was diminished after stimulation, the amount of cytoplasmic calcium increased slightly. These displacements lead to the conclusion that calcium ion influxes, as well as potassium and chloride ion effluxes are involved in the propagation of action potentials. The main difference between the propagation of action potentials in animals and plants is that in an axon there is the potassium/sodium ion transmembrane transport, but in phloem cells the potassium/calcium ion channels are involved in this process (Figure 6b).
 Measuring of action and variation potentials in plants
Measuring of action and variation potentials in plants
Figure 7 is an example of the experimental configuration for the measuring of extracellular action potentials and variation potential. All electrochemical measurements are conducted at constant temperature inside a Faraday cage mounted on a vibration-stabilized table in a laboratory. A microcomputer with multifunction data acquisition board is interfaced, through a Simultaneous Sample and Hold interface with nonpolarizable, reversible silver/silver chloride electrodes to record the digital data. The multifunction data acquisition board provides high resolution and a wide gain range. It features continuous, high-speed data acquisition; a single channel can be sampled at any gain up to 333 ksamples/sec. Each analog input channel has its own instrumentation amplifier with differential inputs. The output of every instrumentation amplifier is routed to a track-and-hold amplifier. In track mode, the outputs of the track-and-hold amplifiers follow their inputs. When put into hold mode, the track-and-hold amplifier outputs simultaneously freeze holding the signal levels constant. The digitized data includes negligible time skew (less than 5 × 10-8 sec) between channels.
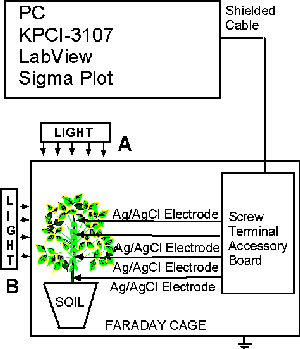 |
| Fig. 7. The experimental set-up for measuring electrical signals in green plants. |
Ksenzhek and Volkov described the preparation of silver/silver chloride electrodes from Teflon coated silver wire. Silver/silver chloride electrodes are sensitive to changes in temperature; therefore, the temperature should remain constant. Both silver/silver chloride electrodes are identical, and are referred to as a reference and working electrodes. The reference electrode (negative) is generally inserted in the stem or in a root of a soybean. On the other hand, the upper working electrode (positive) is inserted in the stem or a leaf. Following insertion of the electrodes, the plants should be allowed to rest until a stable potential difference is obtained between the working and reference electrodes. The insertion of the electrodes in plants induces action potentials across the stem and slow fluctuations of the variation potential. After approximately one to two hours, the variation potential stabilizes.
The automatic measurements of the extracellular and intracellular electrical potential difference can be effectively used in plant electrophysiology to study the molecular interfacial mechanisms of ion transport, the influence of external stimuli on plants, and for investigating the bioelectrochemical aspects of the interaction between insects and plants.
Green plants interfaced with a computer through data acquisition systems can be used as fast biosensors to: monitor the environment; detect effects of pollutants, pesticides, and defoliants; predict and monitor of climate changes; and facilitate the direct and fast control of conditions influencing the agricultural harvest. The use of new computerized methods provides opportunities for detection of fast action potentials in green plants in real time.
 Electrochemistry of photosynthetic systems
Electrochemistry of photosynthetic systems
 Photosynthetic systems
Photosynthetic systems
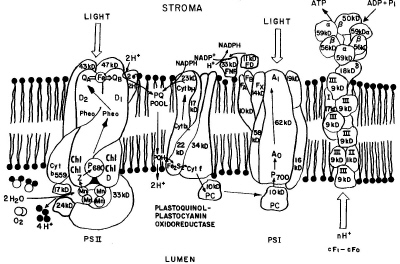 |
| Fig. 8. A schematic model of the electron transport chain with most of the light-harvesting pigment-protein complexes omitted. |
 Structure and composition of the oxygen-evolving complex in vivo
Structure and composition of the oxygen-evolving complex in vivo
The redox map of photosynthesis in green plants can be described in terms of the well-known Z-scheme proposed by Hill and Bendall. The diagram in Figure 9, often called the Z scheme because of its overall form, outlines the pathway of electron flow between two photosystems and the energy relationships in the light reactions. The main advantage of the currently accepted Z-scheme, depicted in Figure 9, lies in the specific mechanism of charge transfer at the stage of water oxidation, which is a multielectron reaction mechanism involving no unknown intermediates.
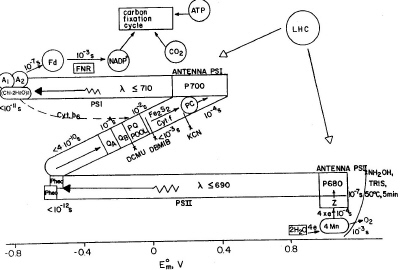 |
| Fig. 9. The electron transfer during photosynthesis in higher plants. The abscissa shows the midpoint redox potential at pH 7.0. Light is absorbed in two sets of antenna chlorophyll molecules, and the excitation energy is transferred to the reaction center chlorophyll a molecules of photosystem II (P680) and photosystem I (P700) forming (P680)* and (P700)*. The latter two initiate electron transport. Abbreviations: LHC is light harvesting complex; Z and D are tyrosine residues; Cyt b559 is cytochrome b559 of unknown function; Pheo is pheophytin; QA, QB and PQ are plastoquinone molecules; Fe2S2 represents the Rieske iron-sulphur center; Cyt f stands for cytochrome f; PC is plastocyanin; Ao is suggested to be a chlorophyll molecule; A1 is possibly vitamin K; FNR is ferredoxin NADP oxidoreductase; x stands for inhibitors (DBMIB: dibromo-3-methyl-6-isopropyl-p-benzo-quinone; DCMU: 3-(3',4'-dichlorophenyl)-1,1-dimethylurea). |
Manganese binding centers were first revealed in thylakoid membrane by electron paramagnetic resonance (EPR) methods, and it is now understood that four manganese ions are essential for oxygen evolution during water photo-oxidation. Plastoquinone (PQ) acts as a transmembrane carrier of electrons and protons between reaction centers of two photosystems in the case of noncyclic electron transfer and may also serve as a molecular "tumbler" that rotates between one-electron reactions and two-electron reactions. Pheophytin is an intermediate acceptor in photosystem II. The direct formation of P680 pheophytin ion radical pairs was revealed by experiments on magnetic interactions between Pheo- and PQ- in the EPR spectra.
Water oxidation to molecular oxygen is a multielectron process that proceeds with surprisingly high efficiency. The water oxidation reaction can proceed upon illumination at 680 nm (billionth of a meter), a wavelength of light that excludes one-electron mechanisms using hydroxyl and oxygen radicals (Figure 11). For a three-electron reaction, an oxidant stronger than the cation-radical P680+ is needed. A synchronous 2:2-electron pathway of the reaction is energetically possible if the energy of the binding of the two-electron intermediate is about -40 kJ/mol. This value corresponds to the energy of two hydrogen bonds forming between hydrogen peroxide and the catalytic center. For this case a molecular mechanism can be proposed (Figure 12), and will be discussed below. The synchronous four-electron oxidation of water to molecular oxygen (Figure 13) is also energetically possible.
One-electron mechanisms of water oxidation are likely to be operative in some model systems with a low quantum efficiency, but two- or four-electron reactions cannot occur due to kinetic limitations. The intermediates formed in these systems would be highly reactive and could enter into side reactions of hydroxylation, oxidation, and destruction of chlorophyll and other components of the reaction center.
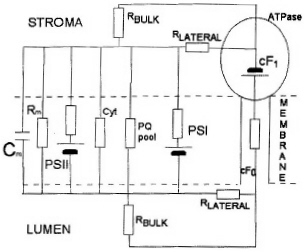 |
| Fig. 10. Electronic equivalent circuit of Photosystems II and I. |
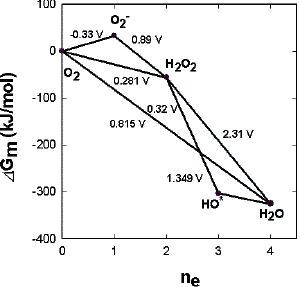 |
| Fig. 11. Energy diagram for possible routes of the reaction 2H2O <==> O2 + 4H+ + 4e-. Gm is the reaction energy at pH 7. |
 Molecular mechanism of oxygen evolution in vivo
Molecular mechanism of oxygen evolution in vivo
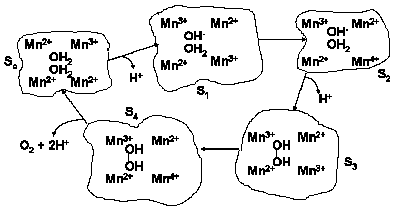 |
| Fig. 12. Proposed 2:2-electron mechanism of water photooxidation. |
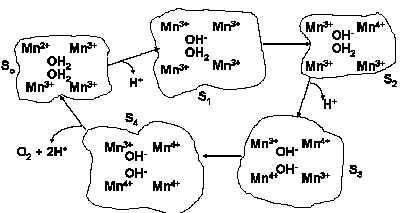 |
| Fig. 13. Proposed four-electron mechanism of water photooxidation. |
Protons are released from reaction centers either by regulators of proton distribution, or by a hydrogen bond transfer through the hydration shell of manganese ions. The hydration sphere of manganese is known to contain water molecules that rapidly exchange protons with bulk water. The presence of divalent cations at the interface between two immiscible electrolyte solutions facilitates strong adsorption of water molecules belonging to the second hydration shell of ions. Thus, a portion of coordinatively bound water enters the compact part of the electric double layer, which changes its differential capacity at the interface. In the case of multivalent ions with small radii, the electric field near a cation is large. This can disturb the microstructure of the adjacent intrathylakoid space and bring about dielectric saturation effects.
Manganese ions play a particularly important role in the evolution of dioxygen during photosynthesis. Although there are several manganese pools in chloroplasts, only one is involved in water oxidation. The manganese ions associated with oxygen evolving complex of the chloroplast can perform a number of functions:
- Mn-polypeptide complex is a redox intermediate that protects the reaction center from redox and radical destruction;
- Mn-clusters are redox buffers facilitating accumulation of four holes in the reaction center of photosystem II, which are needed to ensure water photooxidation;
- Hydrated multivalent manganese cations bring water to the reaction center so that rapid proton exchange and transport through the hydration shell of manganese ions in the zone of water oxidation;
- Multivalent manganese ions induce dielectric saturation effects in the polar region of the reaction center of photosystem II, which reduces the reorganization energy of the medium during charge transfer.
Glossary
Brief definitions of biological terms which are not appropriate for inclusion in the Electrochemistry Dictionary.all-or-nothing law: a statement concerning the reaction of phloem. A stimulus of any intensity that produces one of two responses: a response of invariable strength or no response at all.
ATP: adenosine triphosphate. A coenzyme of fundamental importance found in the cells of all organisms; it provides a means of storage of energy for many cellular activities.
axon: The threadlike extensions on a neuron, or nerve cell which conducts nerve impulses. A neuron usually has one long axon, which branches only at its termination.
biosensor: a device that detects, records, and transmits information regarding a physiological change or process.
Calvin cycle: a light independent set of reactions occurring in the stroma of the chloroplast that uses NADPH and phosphorous from ATP to produce glucose.
chorophyll: any of a group of green pigments found in photosynthetic organisms.
chloroplast: plastid containing chlorophyll.
companion cell: in flowering plants, a cell closely associated in origin, position and function with a cell of a sieve-tube. A companion cell has a dense protoplasm and prominent nucleus. A companion cell and a sieve-tube cell are formed by longitudinal division of one parent cell. It appears to regulate the activity of a sieve-tube.
cotyledon: embryonic leaf in seed-bearing plants.
cyanobacterium: a photosynthetic bacterium of the class Coccogoneae or Hormogoneae, generally blue-green in color and in some species capable of nitrogen fixation. Cyanobacteria were once thought to be algae. Also called blue-green alga. the protoplasm of a cell excluding the nucleus.
cytoplasm: the protoplasm of a cell excluding the nucleus.
dedrite:A long extension of a neuron with thin, treelike branches. It receives nerve signals and transmits them to the main body of the cell
DNA: a long linear polymer found in the nucleus of a cell, shaped like a double helix; associated with the transmission of genetic information.
excitability: the disposition of a tissue or living cell to respond to a stimulus or change in the environment.
excitation: the act of producing or increasing stimulation; the immediate response of a cell or a tissue to a stimulus or change in the environment.
grana: stacks of thin layers in a chloroplast in which the green pigment chlorophyll is contained.
impulse: the electrochemical transmission of a signal along a phloem or a nerve fiber that produces an excitatory or inhibitory response at a target tissue.
ionophore: any of a group of organic compounds that facilitate the transport of ions across the cell membrane.
intercellular: between or among cells.
intracellular: describes a processes occurring, or substance found inside the cell.
ionosphere: the upper region of the Earth's atmosphere, between 50 and 400 km above the Earth's surface. It contains free electrons originating from the ionization caused by ultraviolet radiation and x rays from the sun. The ionosphere reflects radio waves, and also radiation from outer space.
irritant: a material causing irritation, especially physical irritation.
messenger RNA: the template for protein synthesis; the form of RNA that carries information from DNA in the nucleus to the ribosome sites of protein synthesis in the cell.
mitochondrial: a spherical or elongated organelle in the cytoplasm of nearly all plant cells, containing genetic material and many enzymes important for cell metabolism, including those responsible for the conversion of food to usable energy.
morphology: the form and structure of an organism or one of its parts.
neuron: a nerve cell; it receives and conducts electrical impulses from the brain. It consists of a cell body, an axon, axon terminals, and dendrites.
nucleus: central part of a living cell.
organelle: part of a cell that is the structural and functional unit.
pathogen: any disease-producing agent (especially a virus or bacterium or other microorganism).
pheophytin: a chlorophyll in which the central magnesium atom has been replaced by two hydrogen atoms. It acts as an early electron acceptor in photosystem II.
phloem: a type of vascular tissue, in flowering plants it contains sieve-tubes and companion cells supported by ground tissue and also flowers. The phloem conducts food materials synthesized by the leaves, to all parts of the plant.
phosphorylation: biochemical reaction in which an organic substrate, such as sugar, is combined with a phosphate ion by enzyme reaction. The phosphorylation of some organic substances produce high-energy bonds in the product, for example ATP; these products are highly reactive under enzymatic activation.
photosynthesis: the complex process in green plants, in which complex organic compounds are synthesized from carbon dioxide and water using energy obtained from the sunlight.
phototropic response: a tropism in response to light. For example, the growth curvature of a stem to light is an example of phototropism.
plasma membrane: the semipermeable membrane that encloses the cytoplasm of a cell.
polysome: a cluster of ribosomes connected by a strand of messenger RNA and functioning as a unit in protein synthesis.
protonophore: any of a group of organic compounds that facilitate the transport of hydrogen ions across the cell membrane.
protoplasm: the jelly-like granular material which comprises the living content of cells; it is a complex mixture of organic and inorganic substances in a state of continuous chemical change.
refractory period: a time period when the state of a membrane is such that it cannot conduct an impulse; this occurs immediately after an impulse has passed.
resting potential: a membrane potential characteristic of a non-conducting, excitable cell, where the inside of the cell is more negative than the outside.
ribosome: an organelle in the cytoplasm of a living cell.
sieve tube: a long tube-like structure consisting of blast cells arranged in a longitudinal row, with the cells interconnected by sieve plates.
sieve plates: in flowering plants the end walls of a blast cell are pierced by numerous pores through which no substance can pass strands of protoplasm. Sieve-tube plates connect blast cells in a series to form a sieve-tube.
subthreshold: (usually a stimulus that is) not strong enough to be perceived or to produce a response.
threshold: alternative term for minimal stimulus. A stimulus to with intensity just sufficient enough to provoke a response.
thylakoid: a circular, fluid-filled sac that forms grana. Photosynthetic pigments are located in thaylakiod membrane.
tropism: an involuntary orienting response.
uncoupler: a wide range of chemically unrelated compounds that inhibit mitochondrial ATP synthesis but are often observed to stimulate the rate of electron transport.
variation potential: the potential difference between phloem cells.
vascular: relating to the tubes which carry blood or liquids in animals and plants.
Acknowledgement
This article was, by permission, translated into Belorussian and posted at: http://blog.1800flowers.com/international/p01-plants-ua/Related articles
Electric fish, electric organ discharges, and electroreception
Photoelectrochemistry
Bibliography
- Interfacial Catalysis, A. G. Volkov, M. Dekker, New York, 2003.
- Plant Electrophysiology: Excitation Waves and Effects of CCCP on Electrical Signaling in Soybean, A. Labady, D'J. Thomas, T. Shvetsova, and A. G. Volkov, "Bioelectrochemistry" Vol. 57, pp 47-53, 2002.
- Soybean Electrophysiology: Effects of Acid Rain, T. Shvetsova, J. Mwesigwa, A. Labady, S. Kelly, D'J. Thomas, K. Lewis, and A. G. Volkov, "Plant Science" Vol. 162, pp 723-731, 2002.
- Waves of Excitation and Action Potentials in Green Plants, A. G. Volkov, T. Shvetsova, and V. S. Markin, "Journal of Biophysics" Vol. 82, p 218a, 2002.
- Electrochemistry of Soybean: Effects of Uncouplers, Pollutants, and Pesticides, A. G. Volkov and J. Mwesigwa, "Journal of Electroanalytical Chemistry" Vol. 496, pp 153-157, 2001.
- Green Plants as Environmental Biosensors: Electrochemical Effects of Carbonyl Cyanide 3-chlorophenylhydrazone on Soybean, A. G. Volkov, A. Labady, D'J. Thomas, and T. Shvetsova, "Analytical Science" Vol. 17, pp i359-i362, 2001.
- Interfacial Electrical Phenomena in Green Plants: Action Potentials, A. G. Volkov and J. Mwesigwa, in "Liquid Interfaces in Chemical, Biological, and Pharmaceutical Applications" pp 649-681, A. G. Volkov (editor), M. Dekker, New York, 2001.
- Plant Electrophysiology: FCCP Induces Fast Electrical Signaling in Soybean, T. Shetsova, J. Mwesigwa, and A. G. Volkov, "Plant Science" Vol. 161, pp 901-909, 2001.
- Electrochemical Signaling in Green Plants: Effects of 2,4-Dinitrophenol on Resting and Action Potentials in Soybean, J. Mwesigwa, D. J. Collins, and A. G. Volkov, "Bioelectrochemistry" Vol. 51, pp 201-205, 2000.
- Green Plants: Electrochemical Interfaces, A. G. Volkov, "Journal of Electroanalytical Chemistry" Vol. 483, pp 150-156, 2000.
- Plant Electrophysiology: Pentachlorophenol Induces Fast Action Potentials in Soybean, A. G. Volkov, D. J. Collins, and J. Mwesigwa, "Plant Science" Vol. 153, pp 185-190, 2000.
- Plant Energetics, O. S. Ksenzhek and A. G. Volkov, Academic Press, San Diego, 1998.
- Action Potentials of Higher Plants not Possessing Motor Activity, A. M. Sinukhin and V. V. Gorchakov, "Biophysics" Vol. 11, pp 966-975, 1996.
- Insect Induced Bioelectrochemical Signals in Potato Plants, A. G. Volkov and R. A. Haack, "Bioelectrochemical Bioenergetics" Vol. 35, pp 55-60, 1995.
- Action Potentials in Maize Sieve Tubes Change Phloem Translocation, J. Fromm and T. Bauer, "Journal of Experimental Botany" Vol. 45, pp 463-469, 1994.
- Evidence for Two Pathways of Phloem Loading, W. Eschrich and J. Fromm, "Physiologia Plantarum" Vol. 90, pp 699-707, 1994.
- Growth Acceleration of Bean Sprouts by the Application of Electrochemical Voltage in a Culturing Bath, Mizuguchi, Y. Watanabe, H. Matsuzaki, Y. Ikezawa and T. Takamura, "Denki Kagaku" Vol. 62, pp 1083-1085, 1994.
- Characteristics of Action Potentials in Willow (Salix viminalis L.), J. Fromm and R. Spanswick, "Journal of Experimental Botany" Vol. 44, pp 1119-1125, 1993.
- Electric Signaling and Systemic Proteinase Inhibitor Induction in the Wounded Plant, D. C. Wildon, J. F. Thain, P. E. H. Minchin, I. R. Gubb, A. J. Reilly, Y. D. Skipper, H. M. Doherty, P. J. O'Donnell, and D. J. Bowles, "Nature" Vol. 360, pp 62-64, 1992.
- Characteristics of Action Potentials in Helianthus-Annus, T. Zavadzki, E. Davis, H. Dziubinska, and K. Trebacz, "Physiologia Planetarium" Vol. 83, pp 601-604, 1991.
- Control of Phloem Unloading by Action Potentials in Mimosa, J. Fromm, "Physiologia Plantarum" Vol. 83, pp 529-533, 1991.
- Electrical Activity and Signal Transmission in Plants: How Do Plants Know?, E. Davies, T. Zawadzki, and D. Witters, in "Plant Signalling, Plasma Membrane and Change of State" pp 119-137, Universite de Geneve,.1991.
- Electric Signals Released From Roots of Willow (Salix viminalis L.) Change Transpiration and Photosynthesis, J. Fromm and W. Eschrich, "Journal of Plant Physiology" Vol. 141, pp 673-680, 1989.
- Transmission of Electrical Signals in Sieve Tubes of Zucchini Plants, W. Eschrich, J. Fromm, and R.F. Evert, "Botanica Acta" Vol. 101, pp 327-331, 1988.
- Electrochemistry of Plant and Signal Transduction, J. A. Raven, in "The Cell Surface in Signal Transduction" Springer, Berlin, 1987.
- The Evolution of Plant Action Potentials, A. Goldsworthy, "Journal of Theoretical Biology" Vol. 103, pp 645-648, 1983.
- Intercellular Communication in Plants: Evidence for a Rapidly Generated, Bidirectionally Transmitted Wound Signal, E. Davies and A. Shuster, "Proceedings of the National Academy of Sciences" Vol. 78, pp 2422-2426, 1981.
- The Role of Electricity in Plant Movements, P. J. Siomons, "New Physiologist" Vol. 87, pp 11-37, 1981.
- Action Potentials and Rapid Plant Movements, T. Sibaoka, in "Plant Growth Substances 1979, Proceedings of the 10th International Conference, Madison, Wisconsin, July 22-26, 1979" pp 462-469, F. Skoog (editor), Springer, Berlin, 1980.
- Action Potentials in Lipinus angustifolius L. shoots. V. Spread of Excitation in the Stem, Leaves and Root, T. Zavadzki, "Journal of Experimental Botany" Vol. 31, pp 1371-1377, 1980.
- Resting Membrane Potential and Action Potential of Nitella expansa Protoplasts, S. Abe, J. Takeda, and M. Senda, "Plant Cell Physiology" Vol. 21, pp 537-546, 1980.
- Electrical Diffuse Layers and Their Influence on Photosynthetic Processes, J. Barber, J. Mills, and A. Love, "FEBS Letters" Vol. 74, pp 174-181, 1977.
- Electro-Osmotic and Biopotential Measurement on Phloem Strands of Nymhoides, D. S. Fensom and D. C. Spanner, "Planta" Vol. 88, pp 321-331, 1969.
- Action Potentials in the Reproductive System of Plant, A. M. Sinukhin and E. A. Britikov, "Nature" Vol. 215, pp 1278-1280, 1967.
- Excitable Cells in Mimosa, T. Sibaoka, "Science" Vol. 137, pp 226-228, 1962.
- Plant Autographs and Their Revelations, J. C. Bose, Macmillan, New York, 1927.
- Transmission of Excitation in Plants, J. C. Bose, "Nature" Vol. 119, pp 48, 1927.
- The Nervous Mechanism of Plants, J. C. Bose, Longmans-Green, New York, 1926.
- Transmission of Stimuli in Plants, J. C. Bose, "Nature" Vol. 115, pp 457, 1925.
- On the Electromotive Properties of Dionaeva in the Excited and Unexcited States, J. Burdon-Sanderson, "Philosophical Transactions" Vol. 179, pp 417-449, 1888.
- On the Electromotive Properties of Dionaeva in the Excited and Unexcited States, J. Burdon-Sanderson, "Philosophical Transactions" Vol. 173, pp 1-53, 1882.
- On the Mechanical Effects and on the Electrical Disturbance Consequent on Excitation of the Leaf of Dionaeva Muscipula, J. Burdon-Sanderson and F. J. M. Page, "Proceedings of the Royal Society of London" Vol. 25, pp 411-434, 1876.
Listings of electrochemistry books, review chapters, proceedings volumes, and full text of some historical publications are also available in the Electrochemistry Science and Technology Information Resource (ESTIR). (http://knowledge.electrochem.org/estir/)
Return to: Top – Encyclopedia Home Page – Table of Contents – Author Index – Subject Index – Search – Dictionary – ESTIR Home Page – ECS Home Page
ECS | Redcat Blog | ECS Digital Library