Return to:
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
INDUSTRIAL ORGANIC ELECTROSYNTHESIS
With Some Advice on Approaches to Scaleup
Norman L. Weinberg
Consultant
East Amherst NY, USA
E-mail: nweinberg@adelphia.net
(May, 2002)
Thousands of electrochemical reactions of organics have been catalogued to date. These comprise direct electron transfer reactions at anodes for oxidation and at cathodes for reduction. Thousands of these also include indirect electron transfer reactions using redox species. Of these, not more than a few hundred pilot and industrial scale organic electrosyntheses have been described (see Tables I-III below for examples).
The essential difference between chemical and electrochemical processing is that the reactor is an electrolytic cell powered by a current source. The electrolytic cell contains positively charged anodes and negatively charged cathodes; an electrolyte solution containing ions to carry the current and in which the reactant and product are usually at least partially dissolved; maybe, separators (membranes or porous diaphragms) to separate the processes at the anodes and cathodes; and, some means for stirring or agitating the cell contents. The electrodes may be made of special catalytic material, that is these may be electrocatalytic coatings with special properties for optimizing the yield, increasing product specificity, extending electrode life, and/or lowering cell voltage. The electrodes are preferably spaced as close together as possible without touching to avoid shorting, so as to minimize the cell voltage (See Economics).The dc power supplies or rectifiers electrify the cell, at relatively low cell voltages usually in the range of about 3 to 15 volts.
There are many other differences seen in organic electrosynthesis compared to conventional organic synthesis. Useful concentrations of highly reactive cation or anion radicals, not easily or so far impossible to make chemically, can be easily and conveniently produced electrochemically. The resulting electrosynthesis products can be unique (that is not before synthesized by chemical means, or so difficultly made by chemical means that many steps would be required). Many other reactive species can be made conveniently, including superoxide ion, hydroxyl radicals, peroxide, CO2 anion radicals, hydrogen atoms and metal hydrides, and halogens, including fluorine. On the cathode side of the cell, at high negative potentials, solutions of solvated electrons can be readily made and on the anode side, at high positive potentials, powerful oxidants like fluorine, persulfate salts, and ozone. Acid can be made at the anode and alkali at the cathode.
Advantages and disadvantages of electrosynthesis
There are advantages and disadvantages to organic electrochemical processing. Most important among the advantages is the very wide range of oxidation and reduction reactions possible. Other advantages that may be realized are: significantly less energy requirement; less hazardous process; elimination or minimization of polluting byproducts requiring disposal; process simplification so that an otherwise multistep chemical route is simplified to one or two steps; use of cheaper more readily available starting materials; the possibility of reaching very high levels of product purity and selectivity; development of valuable intellectual property; and, in many instances, considerably improved capital and operating costs over conventional methods.
Electrosynthesis certainly has disadvantages too. Electrosynthesis usually requires the use of a solvent to solubilize the reactants and products. Water is the ideal solvent but too often organic solvents or co-solvents are required. In addition, supporting electrolytes to carry the current are very often needed. The solvent/supporting electrolyte system can be too expensive or even the source of unacceptable pollutants if not recovered and recycled. Electrolytic cells require stable electrode materials, separators and other components, which may have limited lifetimes and can affect the economics adversely. Electricity is required in all electrochemical processing which may or may not be a critical factor, depending on where the process is located. (Note, however, that the cost of electricity is not at all a deciding factor where higher value added products such as pharmaceuticals are the products).
Considering the advantages, critics question why there are so few commercial scale organic electrosyntheses. Indeed, there are many ongoing successful processes (See Tables I-III), but as in conventional processes, some have been discontinued or may never reach commercial scale for various reasons, including:
- The product became a pollutant (for example tetraalkyl lead from lead and alkyl halide);
- The market for the product disappeared (for example 1,2-dihydrophthalic acid);
- An established market for the products never existed (for example Philip's electrofluorination of hydrocarbons). A new process for a new product is bound to have a tough upward battle to achieve market entry;
- Toxic byproducts could not be eliminated (for example p-aminophenol produced from nitrobenzene is contaminated with carcinogenic benzidine);
- Improved methods of catalytic hydrogenation became more practical (for example sorbitol from glucose or aniline from nitrobenzene);
- Methods of catalytic oxidation became more practical (for example hydroquinone from benzene or phenol or propylene oxide from propylene);
- The capital cost is considered too high for the company in its present financial state.
- Courage! For various reasons, the product or process has no upper level corporate "champion" willing to support it, despite the promising opportunity and economics;
- Politics! If the company manufactures this product, the company perceives it may lose an important customer or partner, who may see this as competition.
It should not be surprising that the above list of reasons why electrochemical processes never reach commercialization or are discontinued pertains to conventional processing as well.
Chemical industry's attraction to electrosynthesis
The advantages cited above are good reasons for companies to be aware of advances in electrochemical processing. There are also good reasons for chemical companies to have on hand at least the basic laboratory tools and the necessary skills to evaluate potential electrochemical routes alongside conventional methods. But, all too often the primary reason why companies investigate electrochemical processing alternatives is because the company has tried every conceivable chemical method available without success. Then someone in the company, perhaps an electrochemist, an external consultant, or a scientist reading the literature has asked if electrosynthesis might be the long-sought answer. In reality, except for rare instances, there are usually no easy electrosynthesis answers, if no chemical route works.
Attaining successful industrial organic electrochemical processes
The importance of adopting a strategic approach cannot be overemphasized, especially where a company does not have the necessary in-house expertise. Reaching an informed decision on whether or not to pursue a proposed electrochemical route requires consideration of all of the following:
- The electrochemical literature, including patents on the electrosynthesis of the product and its nearest analogues.
- For the most promising route, write the equations for the anode and cathode processes, including products and byproducts. Then decide what needs to be demonstrated in the laboratory in a limited series of studies during small scale feasibility testing. Usually some electroanalytical method such as cyclic voltammetry is useful to follow the electrooxidation or electroreduction of the reactants. Calculate the cell voltage (if not indicated in the literature) and estimate the cell requirements, the electrode area and the number of cells. And, do at least a first pass economic evaluation of capital and operating costs.
- If not already performed, compare the economics of the most promising chemical route. Based on the economics, decide on what variables, if any, are likely to be critical in the electrochemical route. Nothing can be done about certain factors like the number of electrons required, the molecular weight of the starting material or products, but the economics may be influenced positively, for example, by increasing the product yield, current efficiency and selectivity, or by lowering the cell voltage, and by the choice of solvent, operating temperature and cell components like electrode and separator materials.
- A limited number of the feasibility experiments are performed in the laboratory using a simple cell. Glass cells and small flow cells are commercially available. Is the desired product formed? What are the yields and current efficiencies of the products and byproducts? If the answers are found acceptable, a series of well designed experiments will normally follow, preferably in a small flow cell with good flow characteristics to begin to optimize some of the critical variables of the early experiments. All along the way, "go/no go" decisions will be made. Further work may be discontinued in a "no go" decision upon discovery of unacceptable byproducts or the requirement of a too expensive solvent.
- Recalculate the economics, based on the results to this point.
- If still promising, the engineering team may come in to produce a preliminary process flow diagram and assist with advice and design of the scale facility.
 |
| Fig. 1. An example of electrosynthesis cells: Progenica™ Cell (Photo reproduced with permission of Regenesys Technologies Limited). |
In many instances, especially for high value added products, pilot scale may be the plant scale. Designing your own electrochemical cell may be unnecessary. Modern cells are highly engineered reactors and are commercially available in a variety of sizes and with a range of cell components (Figure 1). The following are a few companies that offer cells:
 — —  ElectroCell
http://www.chematur.se/ ElectroCell
http://www.chematur.se/
 — —  IneosChlor
http://www.ineoschlor.com/ IneosChlor
http://www.ineoschlor.com/
 — —  Progenica
http://www.electrosynthesis.com/ Progenica
http://www.electrosynthesis.com/
 — —  C-Tech
http://www.capenhurst.com/ C-Tech
http://www.capenhurst.com/
- Successful pilot scale evaluation of electrochemical processes initially requires set up of the electrolytic cell, power supply, pumps, flow meters, piping, and controls to adequately simulate what is expected for the plant. The immediate objectives of piloting are proving that the process is viable at larger scale and for longer continuous running. A a rule of thumb, the initial scale can be as little as say production of 1/4 to 1 kg/day of product or an order of magnitude higher. The minimum continuous running time for deeming a process technically promising is 1000 hours. During that period, investigators look for sustained high product yields, purity, and current efficiencies. Any surprises in byproduct formation, stability of cell components, especially electrodes and membranes, increasing cell voltage, etc. may cause reassessment of the process. Experience shows that most problems begin to show up within 1000 hours of operation. The next section delves into engineering considerations critical to the success of the process.
Engineering considerations
After successful R&D/preliminary economics, the optimization of the commercial process requires attention to:
 Mass transfer Mass transfer
The rate of mass transfer of reactants and products across the boundary layer at the electrode can be improved by:
- Rotation of a cylindrical or disc electrode: capital investment high
- External pumping of electrolyte between the electrodes, with or without turbulence promoters
- Turbine or propeller agitators
- Fluidized bed of electrode particles: capital investment high
- Fluidized bed of non-conducting particles
- Vibration of the working electrode: capital investment high
- Gas sparging
The majority of commercial electrochemical cells are parallel plate designs with:
(a) external electrolyte pumping, or (b) gas sparging
 Heat transfer Heat transfer
Removal of heat generated within cells is usually required for maintaining the optimum electrolysis temperature. This is accomplished by:
- Circulation of electrolyte through external heat exchangers: the most predominant method
- Internal cooling of the cell via coils, jackets or tubes
- Internal or external evaporative cooling
Factors in sizing the total heat removal load include calculation of:
- Electrolysis power input
- Heats of reaction of main and side reactions
- Heat input of electrolyte pumps
- Heat removed from: humidification of off gasses, evaporation of solvent, reactant, products, or by products
- Heat required to bring the electrolysis system feed stream to operating temperature
 Power usage Power usage
The power usage is a function of cell voltage and current efficiency, but it will be also influenced by the current leakage in bipolar cells, parasitic electrode reactions, pumping losses, and rectifier losses.
 Electrode life Electrode life
The corrosion, passivation, and fouling of both anodes and cathodes can only be evaluated after many months of testing. Electrode life can be greatly affected by:
- Electrochemically induced corrosion
- Chemical reaction with solvent, products, or byproducts
- Too high an operating current density
- Mechanical breakage resulting from:
- high stresses in a cell
- internal gas pocketing, especially in porous electrode materials, such
as carbons
- handling, reuse and long term "wear and tear"
- Fouling from corrosion products, electrolyte impurities, products, and byproducts
 Separator life Separator life
The performance of separators, whether porous diaphragms or ion exchange membranes can only be evaluated after many months of testing. Separator materials can be greatly affected by:
- Chemical reaction with solvent, products, or byproducts
- Dissolution in solvent, products, or byproducts
- Too high an operating temperature
- Too high an operating current density
- Mechanical breakage resulting from:
- unbalanced electrolyte pressures
- unsupported membranes under turbulent flow conditions
- internal gas pocketing
- handling, reuse and long term "wear and tear"
- Fouling from corrosion products, electrolyte impurities, products, and byproducts
 Solvent/supporting electrolyte quality Solvent/supporting electrolyte quality
The buildup of impurities from corrosion and reaction byproducts can only be evaluated after months of pilot testing. Such byproducts can be severely detrimental to overall process performance and optimal performance of cell components. To rectify this situation, it may be necessary to provide for a purge stream or solvent/ supporting electrolyte clean-up step to increase quality.
The economics for the electrochemical portion of the process is governed by operating and capital costs as presented in more detail in the Appendix. For the best economics, it can be seen that low cell voltage, high current density, high current efficiency, and high product selectivity are needed.
Examples of commercial scale and piloted organic electrochemical processes
Tables I-III below list typical examples of on-going commercial scale processes, piloted processes which have not reached commercialization, and processes which had been commercialized but discontinued for various reasons. More exhaustive compilations can be found in the literature. In addition there are many more electrosynthesis processes, especially of fine chemicals, which are kept proprietary and are not found in the open literature.
Two systems are selected as most interesting examples:
- The production of adiponitrile (ADN) is important because this compound in used in the production of the well-known plastic: nylon. The electrohydrodimerization of acrylonitrile (ACN) to adiponitrile is the largest scale commercial electroorganic process, with an estimated total production worldwide of about 340,000 metric ton/year. Discovered by Manuel Baizer in the early 1960's, the "Monsanto Process" was relatively quickly brought to commercialization by a team headed by Donald Danly. Their equipment, cell designs and cell parts such as membranes and electrode materials were primitive by modern standards. Baizer's key innovation was discovery that quaternary ammonium salts such as tetraethylammonium p-toluenesulfonate greatly increased the yield and current efficiency of the conversion of acrylonitrile to adiponitrile (see chemical equation in the Appendix).
The modern-day process uses cadmium cathodes and steel anodes in a bipolar cell containing no membranes, with a two phase recirculating aqueous emulsion of ACN, ADN, a bisquaternary salt (hexamethylene(bisethyltributyl)ammonium phosphate), phosphate buffer, and the anode anticorrosion additives, borax and EDTA. The process is conducted at 55oC (131oF) and a current density of 2 kA/m2. A fraction of the organic phase is continuously removed from the emulsion reservoir for separation of the product. The aqueous phase also is treated continuously to prevent accumulation of organic byproducts and metallic salts from electrode corrosion.
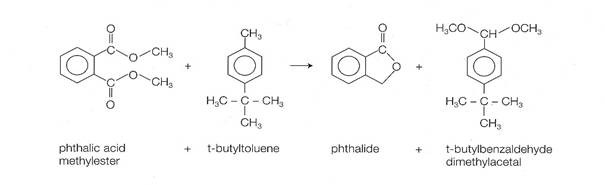
- An excellent example of an innovative industrial organic electrosynthesis process is the following. This process was developed at BASF and was described by Hermann Putter in 1999, at the 13th International Forum on Applied Electrochemistry. This paired 4000 metric ton/year process yields two valuable products simultaneously, phthalide and t-butylbenzaldehyde, by electrolysis in an undivided bipolar capillary gap cell with graphite electrodes (see chemical equation in the Appendix). Other important features of this process are:
- This is the first commercial example of a paired organic
electrosynthesis (useful products are produced both on the
anode and on the
cathode).
- Methanol is both a reagent and a solvent: as much methanol is released
from reduction of the diester as is consumed in making
t-butylbenzaldehyde.
- Current density is 0.1 to 1
kA/m2 at a
cell voltage of 4
to 7 volts.
| Table I. Commercial processes |
| Product | Starting material | Company |
| Acetoin | Butanone | BASF |
| Acetylenedicarboxylic Acid | 1,4-Butynediol | BASF |
| Adipoin Dimethyl Acetal | Cyclohexanone | BASF |
| Adiponitrile | Acrylonitrile | Monsanto (Solutia), BASF, Asahi Chemical  |
| 4-Aminomethylpyridine | 4-Cyanopyridine | Reilly Tar |
| Anthraquinone | Anthracene | L. B. Holliday, ECRC |
| Azobenzene | Nitrobenzene | ? |
| Bleached Montan Wax | Raw Montan Wax | Hoechst |
| Calcium Gluconate | Glucose | Sandoz, India |
| Calcium lactobionate | Lactose | Sandoz, India |
| S-Carbomethoxymethylcysteine | Cysteine + Chloroacetic Acid | Spain |
| L-Cysteine | L-Cystine | Several |
| Diacetone-2-ketogulonic Acid | Diacetone-L-sorbose | Hoffman-LaRoche |
| Dialdehyde Starch | Starch | India, Others |
| 1,4-Dihydronaphthalene | Naphthalene | Hoechst |
| 2,5-Dimethoxy-2,5-dihydrofuran | Furan | BASF |
2,5-Dimethoxy-2,5-dihydrofuryl-1-ethanol  | Furfuryl-1-ethanol | Otsuka |
| Dimethylsebacate | Monomethyladipate | Asahi Chemical |
| Gluconic Acid | Glucose | Sandoz, India |
| Hexafluoropropyleneoxide | Hexafluoropropylene | Hoechst |
| m-Hydroxybenzyl Alcohol | m-Hydroxybenzoic Acid | Otsuka |
| Mucic Acid | Galacturonic Acid | EDF |
| Perfluorinated hydrocarbons | Alkyl substrates | 3M, Bayer, Hoechst |
| Phthalide + t-Butylbenzaldehyde Acetal | Dimethyl Phthalate + t-Butyltoluene  | BASF |
| p-Methoxybenzaldehyde | p-Methoxytoluene | BASF |
| Polysilanes | Chlorosilanes | Osaka Gas |
| p-t-Butylbenzaldehyde | p-t-Butyltoluene | BASF, Givaudan |
| Salicylic Aldeyde | o-Hydroxybenzoic Acid | India |
| Succinic Acid | Maleic Acid | CERCI, India |
| 3,4,5-Trimethoxybenzaldehyde | 3,4,5-Trimethoxytoluene | Otsuka Chemical |
| 3,4,5-Trimethoxytolyl Alcohol | 3,4,5-Trimethoxytoluene | Otsuka Chemical |
| Table II. Piloted processes/not yet commercialized |
| Product | Starting material | Company |
| 1-Acetoxynaphthalene | Naphthalene | BASF |
| Acetylenedicarboxylic Acid | 2-Butyne-1,4-diol | BASF |
| 2-Aminobenzyl Alcohol | Anthranilic Acid | BASF |
| Anthraquinone | Naphthalene, Butadiene | Hydro Quebec |
| Arabinose | Gluconate | Electrosynthesis Co. |
1,2,3,4-Butanetetracarboxylic Acid  | Dimethyl Maleate | Monsanto |
| Ceftibuten | Cephalosporin C | Electrosynthesis Co., Schering Plough  |
| 3,6-Dichloropicolinic Acid | 3,4,5,6-tetrachloro-picolinic Acid  | Dow |
| Ditolyliodonium Salts | p-Iodotoluene, Toluene | Eastman Chemical, Electrosynthesis Co. |
| Ethylene Glycol | Formaldehyde | Electrosynthesis Co. |
| Glyoxylic Acid | Oxalic Acid | Rhone Poulenc, Steetley |
| Hydroxymethylbenzoic Acid | Dimethyl Terephthalate | Hoechst |
| Monochloroacetic Acid | Tri- and dichloroacetic Acid | Hoechst |
| Nitrobenzene | p-Aminophenol | India, Monsanto |
| 5-Nitronaphthoquinone | 1-Nitronaphthalene | Hydro Quebec |
| Partially Fluorinated Hydrocarbons | Alkanes and Alkenes | Phillips Petroleum |
| Pinacol | Acetone | BASF, Diamond Shamrock |
| Propiolic Acid | Propargyl Alcohol | BASF |
| Propylene Oxide | Propylene | Kellog, Shell |
| Substituted Benzaldehydes | Substituted Toluenes | Hydro Quebec, W.R. Grace |
| Table III. Discontinued commercial processes |
| Product | Starting material | Company |
1,2-Dihydrophthalic Acid  | o-Phthalic Acid | BASF |
| 2-Methyldihydroindole | 2-Methylindole | L. B. Holliday, BASF  |
| Hexahydrocarbazole | Tetrahydrocarbazole | L. B. Holliday, BASF |
| Piperidine | Pyridine | Robinson Bros. |
| Sorbitol | Glucose | Hercules |
| Tetraalkyl Lead | Alkyl Halide, Pb (anode)  | Nalco |
Future trends
Based on the past, one can comfortably predict that the future of industrial organic electrochemical processing is promising. Certainly discovery and commercialization of high value added products will continue and at a much lesser pace some inroads will be made towards electrochemical manufacture of commodity chemicals so that adiponitrile does not stand as the only example of large-scale production. Perhaps the prize will be electrosynthesis of ethylene glycol or an olefin oxide like propylene oxide. With the advent of less expensive cells and cell components and discovery of new electrocatalytic materials new opportunities could arise that would have been impractical years before. For example, it has been speculated that one day commercialization of an electroenzymatic process will occur. This would be based on the application of enzymes as catalysts, dissolved in the electrolyte solution or fixed to a support, such as an electrode or separator. The goal would be to harness the extraordinary abilities of enzymes to produce highly valuable, unusual products, perhaps having high stereoselectivity. Realization of an industrial scale electroenzymatic process may take many years to fulfill, but work is already underway in a number of laboratories worldwide to one day achieve this goal.
There are at this writing no academic institutions where courses in applied electrochemistry are taught. In the USA, the closest disciplines taught are electroanalytical chemistry
and electrochemical engineering. Fortunately, there is a large literature of resource materials, including excellent books (see Bibliography). Electrochemical Society meetings (http://www.electrochem.org) and other international symposia, such as the Annual International Forum on Applied Electrochemistry (http://www.electrosynthesis.com) are excellent meetings to explore ideas and find assistance from experts in the field.
What is available also to help those interested in applied organic electrosynthesis are many commercial cells of flexible design; stable cell components, including catalytic electrodes, highly selective membranes and a number of novel electrode/membrane composites; a sound theoretical and practical knowledge base in electrochemistry and electrochemical engineering; and, experienced groups that can advise on R&D, engineering, plant design, construction, and start-up.
Appendix
 Electric power consumption Electric power consumption
K = ( 100 Ko n F V ) / ( Mp Ec )
 Cell costs Cell costs
A = ( 1.12 × 103 n P ) / ( Mp i Ec )
where:
To calculate an approximate cell cost, using the above determined electrode area requirement, use $10,000/m2 for larger installations (say, >10 m2) and $15,000/m2 for smaller installations (<10 m2). Theses figures include electrodes (anodes and cathodes), membranes, frames, spacers, gaskets or o-rings, end-plates and cell fittings for electrolyte, and electrode connections. Contact the cell manufacturer to determine an exact cell cost. For the best economics, it can be seen that low cell voltage, high current density, high current efficiency, and high product selectivity are needed.
 Conversion of acrylonitrile to adiponitrile Conversion of acrylonitrile to adiponitrile
2 CH2 = CHCN + 2 H + + 2 e - ==>
NC-CH2CH2CH2CH2-CN
 Simultaneous production of phthalide and t-butylbenzaldehyde Simultaneous production of phthalide and t-butylbenzaldehyde
Related articles
Aluminum production
Brine electrolysis
Current density distribution in electrochemical cells
Extracting metals from sulfide ores
- Organic Electrochemistry (4th edition), H. Lund and O. Hammerich (editors), Marcel Dekker, New York, 2001.
- Perspectives: The Last 15 Years and Beyond, N. L. Weinberg in "Proceedings of the 15th Annual Forum on Applied Electrochemistry" Electrosynthesis Company, Lancaster NY, 2001.
- Electrolytic Processes–Present and Future Prospects, Electrosynthesis Company and Dextra Associates, Report Number TR-107022, EPRI, Palo Alto CA, 1997.
- Principles of Electrochemical Engineering and Scaleup, D. E. Danly in "Proceedings of the 5th Annual Forum on Applied Electrochemistry" Electrosynthesis Company, Lancaster NY, 1991.
- Industrial Electrochemistry (2nd edition), D. Pletcher and F. C. Walsh, Chapman and Hall, London, 1990.
- Electrosynthesis–From Laboratory to Pilot, to Production, J. D. Genders and D. Pletcher (editors), Electrosynthesis Company, Lancaster NY, 1990.
- Proceedings of the Annual Forum on Applied Electrochemistry (15 volumes), Electrosynthesis Company, Lancaster NY, since 1987.
- Emerging Opportunities for Electroorganic Processes, D. E. Danly, Marcel Dekker, New York, 1984.
- Techniques of Electroorganic Chemistry (Part III), N. L. Weinberg and B. V. Tilak (editors), Wiley Intersciene, New York, 1982.
- Workshop on the Status of Industrial Organic Electrochemistry, SRI International, EPRI EM-2173, Project 1086-9, Research Reports Center, Box 50490, Palo Alto CA, 1981.
- A Survey of Organic Electrolytic Processes, T. R. Beck, R. Alkire, and N. L. Weinberg, DOE Contract Number 31-109-4209, Report No. ANL/OEPM-79-5, Argonne National Laboratory, Argonne IL, 1979.
Listings of electrochemistry books, review chapters, proceedings volumes, and full text of some historical publications are also available in the Electrochemistry Science and Technology Information Resource (ESTIR). (http://knowledge.electrochem.org/estir/)
Return to:
Top –
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
|
|


