Return to:
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
PILLARS OF MODERN ELECTROCHEMISTRY: A BRIEF HISTORY
Ashok K. Shukla and T. Prem Kumar
Central Electrochemical Research Institute
Karaikudi, India
(November, 2008)
Although there is some archaeological evidence
which suggests that some form of a primitive
battery (sometimes called a Baghdad battery) was
used for electroplating in Mesopotamia ca. 200 BC, electrochemistry as we know it today had its genesis
in the pile of crowns of Alessandro Volta in 1800. The inspiration for his studies might have come from the
famous frog leg experiments of Galvani, who, however,
was content to conclude that the phenomenon was of
biological origin. A metamorphosis took place with
seminal contributions from John Daniell and Michael
Faraday. From such humble beginnings, electrochemistry
today has matured into a multidisciplinary branch of
study. Built on the precision of physics and depth of
materials science, it encompasses chemistry, physics,
biology, and chemical engineering.
The uniqueness of electrochemistry lies in the fact
that the application of a potential or electric field can
help overcome kinetic limitations at low temperatures. Moreover, electrochemical processes can be tuned
to obtain chemically specific products. Electrochemical reactions are also
sensitive to electrode-surface characteristics and electrolyte composition, which opens up several analytical and characterization avenues. Like many
forward thinkers who have strived to make life easier
for us to live, history pages are littered with the names,
some of them long forgotten, of those who have made
electrochemistry what it is today. This article is an
attempt to provide a glimpse of these pillars of
electrochemistry through their contributions.
Birth pangs
It was only in the sixteenth century
that electricity began to be understood.
The English scientist William Gilbert
(1544-1603), known as the �father of
magnetism� for his work on magnets,
was among the first to experiment with
electricity. He devised methods to produce
as well as strengthen magnets. The first
electric generator was constructed by the
German physicist Otto von Guericke
(1602-1686) in 1663. The device generated
static electricity by friction between a large
sulfur ball and a pad. By the mid-1700s
the French chemist Charles Francois de
Cisternay du Fay (1698-1739) discovered
two types of static electricity. He found
that like charges repel each other while
the unlike charges attract. Moreover, he
suggested that electricity consisted of two
fluids: a vitreous form (from the Latin
vitrum for glass) or positive electricity; and
a resinous form or negative electricity.
Later in the century, the two-fluid theory
of electricity was opposed by the one-fluid
theory of Benjamin Franklin (1706-1790).
In 1781 Charles-Augustin de
Coulomb (1736-1806) propounded the
law of electrostatic attraction. �Coulomb�,
the unit of electrical charge, is named in his honor.
 |
| Gilbert |
 |
| Guericke |
 |
| Coulomb |
It was at this time, when insights into the new phenomenon
of electricity were growing, that electrochemistry had its
birth pangs with the Italian physician and anatomist Luigi
Galvani (1737-1798) proposing what he called �animal
electricity�. In a 1791 essay titled De Viribus Electricitatis in Motu
Musculari Commentarius, Galvani proposed that animal tissue
contained an unknown vital force, which activated nerves
and muscles when touched with metal probes. According to
Galvani, animal electricity was a new form of electricity in
addition to the natural electricity produced by lightning (or
by the electric eel and torpedo ray) and the artificial static
electricity produced by friction. The idea of an animal electric
fluid was rejected by Alessandro Volta, who argued that
frog�s legs responded to differences such as metal temper and
composition. However, Galvani stood by his version and even
demonstrated muscular action with two pieces of the same
material.
Interestingly, Galvani�s experiments at the University of
Bologna on the physiological action of electricity involved
not only live frogs but also frog legs that had been detached
from the body. He showed that muscle contractions in frogs
and other animals could be triggered by an electric current from a Leyden jar or a rotating static electricity generator.
The twitching of the frog�s legs marked the experimental
phenomenon that has come to be known as bioelectrogenesis.
In fact, Galvani�s experiments not only helped establish the
basis for the biological study of neurophysiology, but also led
to a conceptual change by acknowledging nerves as electrical conductors rather than as mere water pipes, as held by the
Descartes school. Galvani�s name came to be associated
with galvanization (a technique of administering electric
shocks, although another term, faradism, was also used for
the technique). The word galvanizing has shed this archaic meaning, and is applied at present to a protective treatment
of steel with zinc. Galvani is also immortalized in the English
word �galvanize�, which means to stir up sudden/abrupt action.
Nineteenth century: the first half
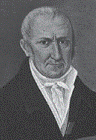 |
| Volta |
The credit for laying the cornerstone
of modern electrochemistry must,
however, go to Alessandro Giuseppe
Anastasio Volta (1745-1827),
a professor of natural philosophy
at the University of Pavia, who showed
in the early 1800s that animal tissue
was not necessary for the generation
of current. He argued that the frog legs
used in Galvani�s experiments served
only as an electroscope and suggested
that the true source of stimulation was
the contact between dissimilar metals.
He called the electricity thus produced metallic electricity.
In fact through his voltaic piles, consisting of alternating
discs of dissimilar metals, he effectively demonstrated the
first electrochemical battery. The epochal invention formed the basis of modern batteries and a host of other galvanic phenomena including corrosion and sacrificial anodes. It also marked the first time that a continuous electric current was
generated. Volta, whose work effectively rejected Galvani�s
animal electricity theory, coined the term galvanism.
Napoleon Bonaparte honored Volta with the title of Count
of Lombardy. Volta is also credited with the discovery and
isolation of methane. Alessandro Volta is immortalized in the
naming of the electrical unit �volt�, a nomenclature that dates back to 1881.
Volta described his invention in a letter dated 20 March
1800 to Sir Joseph Banks (1743-1820), then President of the
Royal Society. It was titled �On the Electricity Excited by the
Mere Contact of Conducting Substances of Different Kinds.�
Banks showed the letter to Anthony Carlisle (1768-1842), a
London surgeon. Enter chemist-engineer William Nicholson
(1753-1815), a friend of Carlisle, and together they assembled
a voltaic pile. In their attempt to determine the charges on the
upper and lower plates with the help of an electroscope, they
put drops of water on the uppermost disc (for better contact!),
and to their surprise found bubbles of gas evolving. Soon they
found that the battery�s terminals dipped in water generated hydrogen and oxygen. They had discovered electrolysis or chemical reaction driven by electric current.
Months later, Johann Wilhelm Ritter (1776-1810)
improved upon the experiments of Carlisle and Nicholson and
created a set-up to collect oxygen and hydrogen separately.
Subsequently, he also invented the process of electroplating. In
fact, Ritter might have made his discoveries earlier than Carlisle
and Nicholson, but could not possibly have published the results
because of his duties as an apothecary. Ritter�s observation of
thermoelectric potential (the electrical potential at the junction
of two dissimilar metals kept at different temperatures) in 1801
also anticipated the 1821 discovery of thermoelectricity by
the Estonian-German physicist Thomas Johann Seebeck
(1770-1831). (Seebeck, however, failed to recognize that an electric current was
generated when a bimetallic junction was heated. In fact, he used the term
thermomagnetic current to describe his discovery. The Seebeck effect forms
the basis of the thermocouple, which is among the most accurate devices for
measuring temperature. The opposite phenomenon, the Peltier effect, which
is the generation of a temperature difference brought about by a current in a
circuit with two dissimilar metals, was observed a decade later.) Ritter�s experiments on the electrical excitation
of muscles included subjecting himself to high voltages, which
might have led to his early death. In this same time period,
English physicist and chemist Henry Cavendish (1731-1810)
made his famous quantitative experiments on the composition
of water and also came out with a version of the Ohm�s law
for electrolyte solutions. He is also known for the famous Cavendish experiment for the measurement of the density of
the Earth. Not too comfortable with publicity, Cavendish lacked
the acclaim that is due to a person of his scientific caliber. In
fact, several of his findings were not published. For example,
he recognized that the force between a pair of electrical charges
is inversely proportional to the distance between them, the
credit for which goes to the French physicist Coulomb. There
are at least two physical structures that should recall him to the
present generation: a square in London, named after him, and
the Cavendish Physical Laboratory at Cambridge University.
 |
| Davy |
The technique of electroplating
was unveiled by Italian chemist Luigi
Brugnatelli (1759-1828) in 1805.
His experiments on gold plating were
performed with a voltaic pile as the
power source. Because he was rebuffed
by Napoleon Bonaparte, Brugnatelli was
forced to keep his results in low profile.
Meanwhile, William Hyde Wollaston
(1766-1828) and Smithson Tennant
(1761-1815), in their attempt to use
electrochemistry to purify platinum,
ended up discovering other elements:
palladium and rhodium (Wollaston) and
iridium and osmium (Tennant). Drawing
inspiration from Ritter, Carlisle, and
Nicholson, Sir Humphrey Davy (1778-1829)
used electrolysis to isolate metals
such as sodium, potassium, calcium,
magnesium, and lithium. He concluded
that electricity induced chemical action
and that chemical combination occurred
between oppositely charged substances.
 |
| Berzelius |
A contemporary and rival of Davy, Jons Jakob Berzelius
(1779-1848) also made important contributions to
electrochemistry. Berzelius found that electrolysis resulted in
the formation of elements at the poles of the cell, which led
him to suggest that atoms were charged and compounds were formed by neutralization of charges. This was his dualism
theory, which, however, did not apply to organic compounds.
Berzelius also established the law of definite proportions. He
is also credited with the discovery of several new elements
including cerium, selenium, and thorium. It was he who
created a logical system of symbols for elements (H, C, Ca, Cl,
O, etc). With the work of Davy and Berzelius, chemistry was
never to be the same again!
 |
| Ampere |
A momentous discovery was made in
parallel by Danish natural philosopher
Hans Christian Orsted (1777-1851),
who observed the magnetic effect
of electric current in 1820. Andre-Marie Ampere (1775-1836), who
took the cue from Orsted, conducted
extensive experiments and formulated
his findings mathematically. Then
came another formulation connecting
voltage, current, and resistance through Ohm�s
law, the work of the German physicist Georg Ohm
(1787-1854) in 1827. Ohm�s discovery was initially ridiculed
by his contemporaries. However, by 1833 the fundamental
importance of Ohm�s law in electrical circuit analysis was
recognized and Ohm came to be considered the Mozart of
electricity.
 |
| Faraday |
Michael Faraday (1791-1867) is
considered to be one of the greatest
scientists in history. Some refer to
him as the greatest experimentalist
ever, especially because his work on
electricity found expression in day-to-day
technology. �Farad�, the unit of
capacitance, and the �Faraday constant�,
are named after him. He invented
the dynamo, predecessor to today�s
electric generator. His concept of
lines of flux emanating from charged
bodies and magnets provided a way to
visualize electric and magnetic fields, and was crucial to the
successful development of electromechanical devices, which
dominated engineering and industry for the remainder of the
19th century. The �Faraday effect�, a phenomenon he named
diamagnetism, was also his discovery. In his work on static electricity, Faraday demonstrated that charge resided only
on the exterior of a charged conductor, and that the exterior
charge had no influence on anything enclosed within a
conductor, a shielding effect we now use in �Faraday cage�.
Faraday worked extensively in chemistry too, discovering
substances such as benzene,
liquefied gases such as chlorine, and proposed the system
of oxidation numbers. He also discovered the laws of electrolysis, by which he quantified electrochemistry, and
popularized terminology such as anode, cathode, electrode,
and ion, terms largely created by William Whewell (1794-1866).
He rejected the traditional fluid theory of electricity
and proposed that electricity was a form of force that passed
from particle to particle in matter.
 |
| Becquerel |
A major problem with the Volta pile
was that it could not provide current
for a sustained period of time. In 1829
Antoine-Cesar Becquerel (1788-1878)
constructed a constant current
cell, which was a forerunner of the
well-known Daniell cell. His acid�alkali
cell could deliver current for an hour.
Becquerel�s studies on electrodeposition
of metals helped validate Faraday�s laws
of electrolysis. The credit for solar cell
technology must be given to Becquerel,
who in 1839 showed that light impinging on an electrode
immersed in a conductive solution would create an electric current. In 1830, William Sturgeon (1783-1850), another
scientist who worked on sustained current generation,
produced a battery with longer life than that of the Volta pile by
amalgamating the zinc. Mercury was found to be a cure for
polarization, a process by which a thin film of hydrogen bubbles formed over the positive electrode. The thin gas film
led to high internal resistance in the Volta pile, resulting in
reduced current flow. In 1832 Sturgeon constructed an electric
motor. The same year witnessed Hippolyte Pixii (1808-1835),
a French instrument maker, build the first dynamo.
Later, using Sturgeon�s commutator, Pixii built a direct current
dynamo, which was the first practical mechanical generator
of electric current.
 |
| Daniell |
In 1836, John Frederic Daniell
(1790-1845) unveiled a two-fluid battery,
which was the first battery to provide a
constant and reliable source of current
over a long period of time. Daniell
used a copper vessel that served both
as the positive pole and the container.
Inside the copper vessel was an earthen
pot with a zinc rod (the negative pole)
and dilute sulfuric acid. The copper
vessel was then filled with a solution of
copper sulfate. The porous pot served
as a barrier, preventing mixing of the
liquids. Although Daniell is famous for his invention of the
two-fluid battery, he is less known for his 1820 invention of
the dew-point hygrometer for the measurement of relative
humidity.
The technique of electroforming was introduced in 1838 by Boris Jakobi (1801-1874). Jakobi applied his technique to
the printing and coinage industries. Soon an electroforming
shop was set up at the Governmental Papers Department,
which was noted for depositing 107,984 kg of copper and 720
kg of gold for decoration of architectural monuments and
cathedrals in St. Petersburg and Moscow.
 |
| Grove |
Sir William Robert Grove (1811-1896)
invented the first fuel cell in
1839. Grove is also credited with the
invention of the Grove�s nitric acid cell:
zinc in dilute sulfuric acid as the anode
and platinum in concentrated nitric acid
as the cathode, separated by a porous
pot. Because the cell could sustain high
current output, it became a favorite with
the early American telegraph industry.
But it was replaced by the Daniell cell
because of the poisonous nitric oxide it
emitted and because of its inability to
deliver currents at constant voltage (the voltage dropped as
the cell discharged due to depletion of nitric acid). However,
his invention of the gas voltaic battery, the forerunner of
modern fuel cells, made him the �father of fuel cells.� In his
experiments that led to the invention, he sought to reverse
the electrolytic splitting of water, to
recombine hydrogen and oxygen
to produce water and electricity.
His background in law and science
opened up the practice of patent and
related laws.
(A note: although the first fuel cell was constructed in 1839, the term �fuel cell� came into vogue only in 1889 with Ludwig Mond and Charles Langer�s attempt to build the first fuel cell using air and industrial coal gas as feed gases.)
 |
| Bunsen |
In 1841, Robert Wilhelm
Eberhard Bunsen (1811-1899) led
the way for large scale exploitation of
the Grove�s cell by replacing the expensive
platinum with a carbon electrode. The modified version was popularly known as the �Bunsen
battery�. To students of science, the name Bunsen is associated
with a burner, although the actual credit for the burner should
go to a technician by name Peter Desaga of the University of
Heidelburg. It must be pointed out that Bunsen fine-tuned the
design of the burner to suit experiments in physics that he
carried out with Gustav Kirchoff, a Prussian physicist. The two
invented the Bunsen-Kirchoff spectroscope.
This was just about the time when the German chemist
Friedrich Wohler (1800-1882) overthrew the vitalism theory
that a vital force was necessary to make organic compounds by
synthesizing urea from ammonium cyanate. Adolf Wilhelm
Hermann Kolbe (1818-1884) also was another chemist
who believed that organic compounds could be made from
inorganic ones. He converted carbon disulfide, an inorganic
compound into acetic acid, an organic compound, in several synthetic steps. Kolbe also made salicylic acid (the Kolbe-Schmitt reaction). Kolbe was the first to apply electrolysis for organic synthesis. He showed that electrolysis of carboxylic acids led to decarboxylation. Loss of carbon dioxide during
the reaction led to dimerization of the resulting alkyl radicals to symmetric compounds (Kolbe synthesis).
During 1842 and 1843, George Gabriel Stokes
(1819-1903) published a series of papers on the motion of
incompressible fluids. They became fundamental to our
understanding of electrolyte solutions. Sometime around 1845,
in what was to mark a revolution in industrial electroplating,
John Wright showed that potassium cyanide was a suitable
medium for plating silver and gold. In 1857 electroplating was
applied to costume jewelry, and soon electroplaters cashed in
on a booming economical jewelry market.
One of the foremost physicists of the nineteenth century,
Prussian-born Gustav Robert Georg Kirchoff (1824-1887),
formulated what are today known as Kirchoff�s laws. When he
announced the laws in 1845, he was still a student, although
in their final forms the laws became known only in 1854. The
laws help in calculating the voltages, currents, and resistances
in multi-loop electrical networks. The laws embody the
principle of conservation of charge and energy. In association
with Robert Bunsen, Kirchoff introduced the spectroscopic
method of chemical analysis, leading in the process to the
discovery of cesium (1860) and rubidium (1861) as well as
unfolding a new technique for discovery of new elements.
Nineteenth century: the second half
In 1853 Johann Wilhelm Hittorf (1824-1914), a
German physicist, noticed that some ions traveled more rapidly than others under an applied current. This finding led
to the concept of transport number. A couple of years later, Adolph Fick (1829-1901), at a mere 26 years, building on the
Fourier�s theory of heat equilibrium, developed a mathematical
concept by which he showed that diffusion is proportional
to concentration gradient. That was in 1855. However,
experimental proof of the concept was not established for the
next 25 years. He was proficient in physiology, mathematics,
and physics, and made another distinctive contribution in the
form of a monograph entitled Medical Physics, in which he dealt
with several topics including mixing of air in the lungs, heat
economy of the body, physiology of muscular contraction,
and circulation. Medical physics had
to wait for nearly a century for another monumental book,
which came through Otto Glasser (1894-1964). Cardiologists
note that Fick made a distinctive contribution in 1870 when
he described how mass balance could be used to measure
cardiac output, thereby presenting a mathematical basis of
physiological activity.
Electrochemists connect the name Josiah Latimer Clark
(1822-1898) with the standard Clark cell used for measuring
the standard electromotive force. This English engineer was a very versatile inventor, known for his work on wireless
telegraphy, particularly the Anglo-American Atlantic cable. It
was Clark who introduced the notion of �volt� as the unit for
voltage. In 1872, Clark invented the first standard cell with mercury and zinc amalgam electrodes in a saturated solution of zinc sulfate. It had large temperature sensitivity, moreover, it was prone to cracking where the platinum lead entered the glass cell. Today, we use the Weston cadmium cell as the standard for the potentiometric measurement of standard electromotive force.
Between 1858 and 1860 the American inventor and
industrialist Isaak Adams, Jr. (1836-1911) pioneered the
technique of nickel plating, which immediately was exploited
on a commercial scale. He was the son of Isaac Adams, Sr.,
the inventor of the Adams power press. Adams also had other
credentials as an inventor: the vacuum-tube carbon burner
incandescent electric bulb, which he invented in 1865, 14
years prior to a similar invention by Edison�Swann; breech-loading
rifles; and copper plating on steel for bonding rubber to steel.
 |
| Plante |
The year 1859 witnessed an invention that was to revolutionize the
world of portable power: the lead-acid battery by the French physicist Gaston Plante (1834-1889). Plante�s storage battery used lead plates as electrodes and delivered limited currents because the positive electrode had very little
of active material. In 1881 Camille Alphonse Faure (1840-1898) replaced
Plante�s solid lead plate with a paste of lead oxide, which led to faster formation kinetics and improved efficiency. The significance of Plante�s
invention can be gauged by the fact that the technology of
the lead-acid battery has changed little since its invention
except for changes in electrode design and casing materials,
and that other battery chemistries are yet to approach the
lead-acid battery in terms of certain electrical capabilities and
economy.
 |
| Leclanche |
Seven years later, in 1866, French scientist Georges
Leclanche (1839-1882) patented a primary cell with a porous
pot containing manganese dioxide and carbon as the positive,
and a zinc rod as the negative. The electrodes were immersed
in an electrolyte of ammonium chloride. The Leclanche wet cell was the
forerunner to the zinc-carbon dry cell which became the world�s first widely
used primary power source. Leclanche�s cell was rendered dry by the German
scientist Carl Gassner (1839-1882), when he cleverly configured the cell
with zinc as a container and negative electrode. Gassner also employed zinc
chloride in the cathode mix so as to reduce the wasteful corrosion of zinc
during idling. The market for dry cells
received a boost with the use of tungsten filament in flashlights in 1909.
Gabriel Lippmann (1845-1921) received the
1908 Nobel
Prize in Physics for inventing the color photographic plate,
but to electrochemists he is associated with the capillary
electrometer, which he invented in 1872. This instrument, based on the extreme sensitivity of mercury meniscus in a
capillary tube to applied potential, was subsequently exploited
for measuring electrocardiograms. His versatility of interests
can be noted in the fact that he was also the inventor of the
coelostat (a long-exposure instrument that allows a region
of the sky to be photographed by compensating for Earth�s
movement) and conducted research in various areas including
piezoelectricity, seismology, and induction in resistance-less circuits. Lippmann was a research advisor to Marie Curie and a professor to Pierre Curie.
Between 1875 and 1879, the
German physicist Friedrich Wilhelm
Georg Kohlrausch (1840-1910)
working with solutions of a variety of
salts and acids developed the law of
independent migration of ions. He was
the first to apply alternating current
for electrochemical investigations. By
using alternating current, he was able
to avoid deposition of decomposition
products on the electrode surface and
obtain results with high precision.
Kohlrausch also demonstrated that ionic
conductivity increased with dilution. He was also noted for
his work on autoionization of water, thermoelasticity, and
thermal conduction and for his precision measurements of
magnetic and electrical properties.
The time was when the electricity was still in its infancy and
the identification of the electron itself was to happen ten years
later. At this time emerged a man who contrived to answer a
question by Maxwell whether the resistance of a coil excited
by an electric current was affected by the presence of a magnet.
Edwin Herbert Hall (1855-1938), an
American physicist well ahead of his
time, discovered what we call the Hall
effect in 1879. The discovery remained
a curiosity for nearly a century until the
emergence of semiconductors that could
produce significant Hall voltages. Today,
Hall effect is used in the primary circuit
of electronic ignition systems.
This was also a momentous period for
the growth of electrochemistry.
The architect of this transformation was
the American scientist Josiah Willard
Gibbs (1839-1903). He was a true genius
and drew on the concepts of great
men like Johannes Diderik van der
Waals (1837-1923) and drew accolades
from peers such as Maxwell.
Interestingly, Yale University�s first
doctoral degree for an engineering thesis
was awarded to Gibbs. He also is known
for his contributions to astronomy and
electromagnetic theory.
 |
| Kohlrausch |
 |
| Hall |
 |
| Gibbs |
 |
| van der Waals |
The nature of electricity came in
for much debate during this period.
The British physicist James Clerk
Maxwell (1839-1871) believed that
electricity was the result of polarization
of the ether or of the medium through
which electric current flowed. However,
in 1881 the German scientist Hermann
von Helmholtz (1821-1894) basing
his theory on Faraday�s laws of electrolysis, which relates the
charge to the amount of metal deposition, argued that the
existence of atoms implied the particulate nature of electricity.
However, proof of the existence of such a particle (electrons)
seemed to contradict Helmholtz theories of electrodynamics,
which were based on the supposed properties of the ether. At
any rate, the discovery of radio waves by Heinrich Rudolf
Hertz (1857-1894), a student of Helmholtz, helped cement
the theories of Faraday, Maxwell, and Helmholtz. Decades
later, Albert Einstein�s general and special theories of relativity
helped dismantle the concept of the pervasive ether.
Maxwell�s successor at King�s College, London, William
Grylls Adams (1836-1915), along with his student, Richard
Evans Day, found in 1876 that selenium upon exposure to
light produced electricity, by a process we recognize today as
photoelectric effect. He thus became the
first to demonstrate that light could be
used to generate electricity without heat
or moving parts.
In his thesis published in 1884,
Swedish physical chemist and the
1903
Chemistry Nobel Prize winner Svante
August Arrhenius (1859-1927)
suggested that dissolving electrolytes
in water resulted in varying degrees of
dissociation of the electrolytes into ions.
The degree of dissociation depended
not only on the nature of the electrolyte
but also on its concentration - the
greater the concentration, the lesser
the dissociation. The concept of
activity coefficient, a quantity that
relates the actual number of ions at
any concentration to their number
upon high dilution, was born out of
Arrhenius�s studies. His collaboration
with Ludwig Eduard Boltzmann
(1844-1906) and Jacobus Henricus
van�t Hoff (1852-1911) led to theories
on the properties of
solutions. Latvian chemist Friedrich
Wilhelm Ostwald (1853-1932), the
1909
Chemistry Nobel Prize winner, extended
Arrhenius�s theory to the electrical
conductivity and dissociation of organic
acids. Ostwald also propounded a theory
of solutions based on ionic dissociation.
In 1884 he came out with a definition
of catalysis. Ostwald is also credited
with the invention of the viscometer.
The application of electrolysis for
winning aluminum from aqueous
solutions of aluminum salts ended up in
the formation of aluminum hydroxide.
However, in 1886 two young scientists,
Paul Louis Heroult in France and
Charles Martin Hall in the United
States (both born in 1863 and died
in 1914), working independently,
succeeded in producing aluminum by electrolysis from
a melt of aluminum oxide in cryolite.
The inventions went through a patch
of patent litigations before Heroult and
Hall agreed to bury their differences.
Their electrolytic process opened up a
new world of industrial applications
for rust-resistant aluminum, which was
until then considered a prized metal
used in fine jewelry.
The theory of electrolytic
dissociation formulated by Arrhenius
inspired another architect of modern
electrochemistry: the 1920
Chemistry Nobel Prize winner Walther Hermann
Nernst
(1864-1941). In 1888, Nernst
came out with a theory connecting
the electromotive force in an
electrochemical cell to the energy
of the chemical reaction that produces
the current. He also demonstrated that
solvents with high dielectric constants
promoted the ionization of substances.
His experiments with solutions led
him to suggest conditions under which
solutes precipitated from solutions.
The theory of solubility product is
also his making. He devised a method
to measure dielectric constant and
demonstrated that solvents with high
dielectric constants facilitated ionization of substances.
Students of physical chemistry know him through the Nernst equation.
 |
| Arrhenius |
 |
| van�t Hoff |
 |
| Ostwald |
 |
| Heroult |
 |
| Hall |
 |
| Nernst |
Tutored by Hermann Kolbe at Marburg and Robert Bunsen
at Heidelberg, Ludwig Mond (1839-1909) became a well
known chemist and industrialist. Initially, his interests were
in the production of elemental sulfur, alkali, and ammonia.
He also developed a process (the Mond process) for the
generation of producer gas. In 1889, he and his assistant
Carl Langer developed a hydrogen�oxygen fuel cell with thin
perforated platinum electrodes. Both Mond and Langer were
instrumental in popularizing fuel cells. In their attempt to use
coal gas as a fuel in fuel cells, they discovered the formation
of nickel carbonyl (the molecules described by Lord Kelvin as
metals with wings) from the carbon monoxide in the coal gas
and the nickel in the electrode. This observation formed the
basis of the Mond process for the extraction of nickel through
a carbonyl route.
 |
| Dow |
In 1890, inventive genius and businessman Herbert
Henry Dow (1866-1930), best known for his work on
halogen chemistry, developed an electrolytic method for
the production of bromine from brine. Today, the method
is known as the Dow process. An offshoot of this work was
an electrolytic production route for chlorine. Slowly, and
with the establishment of the Dow
Chemical Company, he ventured into
chlorine chemicals, organics, and later
into magnesium metal and calcium. By
capitalizing on the brine sourced from
the ancient seas in the Midland region
of the U.S., Dow opened avenues for
mining sea resources.
 |
| Weston |
The first to record a human
electrocardiogram with the Lippmann�s
mercury capillary electrometer was
Augustus Desire Waller (1856-1922),
the French physiologist who
in 1887 thought up the idea of
using the body itself as an electrical
conductor. (The first human electrocardiogram was recorded by Alexander Muirhead
(1848-1920), but it was Waller who did it in a clinico-physiological
setting. Moreover, Muirhead used a Thompson siphon recorder for his
measurements while Waller used Lippmann�s capillary electrometer.)
Willem Einthoven
(1860-1927) improved upon Waller�s
experiments and instrumentation. In
1893, Edward Weston (1850-1936)
developed a standard cell that was to
become the international standard
for the calibration of voltmeters. The
cell was less sensitive to temperature
fluctuations than the previous standard,
the Clark cell, and had the additional
advantage of possessing a voltage very
close to one volt: 1.0183 V.
 |
| Haber |
Based on his work on electrolysis,
the 1918
Chemistry Nobel Prize winner Fritz Haber (1868-1934) in 1898
showed that different products could
be obtained by maintaining the
potential of the electrode at different
values. He explained his findings with
nitrobenzene, which became a model
compound for other investigators.
He also worked on the quinone-hydroquinone transformation, which
became the basis for Biilmann�s quinhydrone electrode for measuring
the acidity of solutions. Haber is also credited with the invention of the glass electrode, a contribution he made with Cremer, one of his associates.
 |
| Edison |
This was also a period that witnessed
as many as 1,093 inventions by a single
person, Thomas Alva Edison (1847-1931),
nicknamed the �Wizard of Menlo
Park.� Although he is most popular for
inventing the incandescent bulb and
the phonograph, his unveiling of the
nickel�iron battery was no less
a contribution. Simultaneously, and
independently of Edison, Waldemar
Jungner (1869-1924) in Sweden
patented the nickel-iron battery. In 1899
Jungner replaced the iron electrode with the more efficient cadmium electrode resulting in the much used nickel-cadmium battery. It is interesting to note that in 1899 the
world record for road speed was held by the electric vehicle!
However, with rapid advancements in internal combustion
engineering, the bottom fell off the electric vehicle market in
the next three decades.
 |
| Tafel |
The year 1898 marked a turning
point in organic electrochemistry with
Swiss chemist Julius Tafel (1862-1918)
demonstrating the use of lead as
an electrode for the reduction of organic
compounds. Tafel, who was both an
organic chemist and a physical chemist,
made seminal contributions to organic
electrochemistry and established the
Tafel equation connecting the rates
of electrochemical reactions and
overpotential. The Tafel equation was
unique in that it could be applied
to irreversible electrochemical reactions that could not be
described in other way. The several contributions
he made in organic chemistry include reduction with
amalgams and the Tafel rearrangement. Tafel is also known
for introducing the hydrogen coulometer
for measurement of electrochemical reaction rates and pre-electrolysis as a
method for purifying solutions.
Twentieth century: first half
Energetic considerations and ionic transport were
the focus of this period. In one of the first approaches to
characterizing aqueous solutions, H. Friedenthal in 1904
suggested the use of hydrogen ion concentrations. In what is
probably the beginning of the concept of pH, Friedenthal also
found that the product of the concentrations of hydrogen
ions and hydroxyl ions in aqueous solutions was always the same. It must be pointed out, however, that the concept of
pondus hydrogenii, or pH, itself was introduced five years later
by the Danish chemist Soren Peder Lauritz Sorensen
(1868-1939).
In what was to mark the beginning of bioelectrochemistry,
Julius Bernstein (1839-1917), of the University of Berlin,
demonstrated that the action electric potential in nerves
was the result of a change in ionic properties of the nerve
membrane. He proposed his membrane hypothesis in two
parts, the first one in 1902 and the second in 1912. His theory
was based on the work of Helmholtz and Du Bois-Reymond
(1818-1896), the �father of experimental electrophysiology�.
Bernstein�s work on the propagation of nerve impulses and
trans-membrane potential led to great interest in bioelectricity
and in the theory of nerve action in particular. It is noteworthy
that Bernstein�s work was the last nail on animal electricity,
and came at a time when electricity was trying to rid itself of
the shadow of biology.
In 1910, Robert Andrews Millikan (1868-1953)
determined the charge on an electron by his famous oil
drop experiments. The following year, Frederick George
Donnan (1870-1956) established the conditions for
equilibrium between two electrolytic solutions separated by a semi-permeable membrane. Today, Donnan�s name is associated with both the nature of the equilibrium and the
potential across the membrane.
 |
| Heyrovsky |
Around 1922, Prague evolved into the �Mecca of electrochemistry.� On
February 10 of that year,
Jaroslav Heyrovsky (1890-1967), sometimes
called the �father of electroanalytical chemistry,� recorded a current-voltage
curve for a solution of sodium hydroxide using a dropping mercury electrode and
ascribed the current jump between �1.9 and �2.0 V to the deposition of sodium
ions on mercury. This marked the beginning of polarography, which took
roots in his early work with F. G. Donnan on the electrode potential of aluminum, leading Heyrovsky into work on
liquid electrodes that provide a continuously renewable
electrode surface. Later, he teamed up with Masuzo Shikata
(1895-1964) and designed the first recording polarograph. In
1959, Heyrovsky was awarded the
Chemistry Nobel Prize for his seminal
work on this electroanalytical technique. Polarography led
to a spurt in the growth of the theory of electrochemical
reactions and mass transport in electrolyte solutions, and laid
the fundamentals of all voltammetric methods employed in
electroanalysis. In 1929, Heyrovsky along with Emil Votocek
of the Prague Technical University, founded the journal,
Collection of Czechoslovak Chemical Communications. Slovakian
physical chemist and mathematical physicist Dionyz
Ilkovic (1907-1980), a research assistant to Heyrovsky, was
one of the co-founders of polarography. The basic equation of
polarography, the Ilkovic equation, goes by his name.
 |
| Debye |
Relationships between molecular structure and electrical
properties were also beginning to be unraveled. In 1923,
Johannes Nicolaus Bronsted (1879-1947) in Denmark
and Thomas Martin Lowry (1874-1936) in England
propounded, independently of each other, a theory on acids
and bases. According to them, an acid was a compound with
a tendency to donate a proton (or hydrogen ion), while a
base was one that combined with a proton. The same year also witnessed
Dutch-American physicist Petrus Josephus Wilhelmus Debye
(1884-1966) and German physicist and physical chemist Erich Armand
Arthur Joseph Huckel (1896-1980) elucidating their fundamental theories
concerning the behavior of strong electrolyte solutions. According to
them, electrolyte solutions deviate from ideal behavior due to ion-ion
attractions. They suggested that ions in solutions have a screening effect on the electric field from individual ions, which gave rise to
the Debye length. Huckel is also famous for the Huckel rule
for determining ring molecules and for the Huckel method
of approximate molecular orbital calculations.
Debye won the 1936 Nobel Prize in Chemistry for his
contributions to molecular structure, for dipole moment
relationships and for diffraction of X-rays and electrons in
gases. In 1916, he showed that X-ray diffraction studies could
be done with powder samples, eliminating in the process the
need to prepare good crystals. This has come to be known as
the Debye�Scherrer X-ray diffraction method. The originality
and extent of Debye�s contributions are reflected in the many
concepts that carry his name: the Debye�Scherrer method
of X-ray diffraction, Debye�Huckel theory, Debye theory of
specific heat, Debye�Sears effect in transparent liquids, Debye
shielding distance, Debye temperature, Debye frequency, and
the Debye theory of wave mechanics. He is also immortalized
by the unit for dipole moment (debye), the Dipole
Moment monument in Maastricht, and the American
Chemical Society award in his name.
 |
| Frumkin |
 |
| Levich |
Around this time, Alexander
Naumovich Frumkin (1895-1976),
popularly known as the �father of
electrochemistry in Russia,� made
vital contributions to our knowledge
of the fundamentals of electrode
reactions � particularly the influence of
the electrode-electrolyte interface on
the rate of electron transfer across it.
Based on his studies on the adsorption
of organic compounds on mercury,
Frumkin proposed an adsorption
isotherm that has come to be known
as the Frumkin isotherm. He also
introduced the concept of potential
of zero charge. He joined hands with
Veniamin Grigorevich Levich
(1917-1987), an associate of theoretical
physicist Lev Davidovich Landau
(1908-1968), in relating his experimental
results to theory. The collaboration led
to the development of the rotating disc
electrode and to a quantitative analysis
of the polarographic maximum.
Electric traction received a boost with
the invention of the so-called Drumm
traction battery. This was a nickel-zinc
alkaline battery invented by James J.
Drumm (1897-1974) and it became
popular with its use in a suburban train
in Ireland. In 1932, Francis Thomas
Bacon (1904-1992) introduced the
use of an alkaline electrolyte and
inexpensive nickel electrode in fuel cells. Twenty-seven years later, in
1959, he demonstrated a practical five-kilowatt fuel cell.
The quantitative measurement
of electrochemical corrosion got
established with a 1932 publication
of Thomas Percy Hoare (1907-1978)
and Ulick Richardson Evans
(1889-1980). Evans described
in the Biographical Memoirs of Fellows
of the Royal Society as the �father of
the modern science of corrosion
and protection of metals�, laid the
foundations of the electrochemical
nature of corrosion. His 1937 book
Metallic Corrosion, Passivity, and Protection
is probably the most comprehensive
book ever written by a single author
on corrosion science. In 1933, in a
paper on the oxygen electrode, Hoare
showed how equilibrium potential
could be determined from Tafel plots.
Hoare was the first recipient of the U.
R. Evans award (1976) of the Institution
of Corrosion Science and Technology.
Herbert H. Uhlig (1907-1993) was
another champion of corrosion science. His Corrosion Handbook
published in 1948 continues to serve
generations of corrosion scientists and
engineers even half a century after its
publication.
 |
| Hoare |
 |
| Evans |
 |
| Uhlig |
 |
| Wagner |
Described by F. Mansfeld as �an
under-appreciated giant in the world
of electrochemistry and corrosion,�
German electrochemist and materials
scientist Carl Wagner (1901-1977) is
also remembered as the �father of solid
state chemistry� for pioneering work in a variety of fields
including tarnishing reactions, catalysis, photochemistry,
fuel cells, semiconductors, and defect chemistry. Wagner
formulated in 1943 the mechanism of ionic conduction in
doped zirconia, which laid the foundations for the field of solid
state ionics. His contributions to corrosion were fundamental
to our understanding of the diffusion-limited growth of scales
on metals at high temperatures as well as of other diverse
aspects such as local cell action, passivity, alloy oxidation,
and cathodic protection. His other contributions include
solid state coulometry, and the theoretical and experimental
aspects of mixed ionic and electronic conduction.
In 1937, Arne Wilhelm Kaurin Tiselius (1902-1971)
turned another page in the history of electrochemistry
with his work on the moving boundary, which was later to
become zone electrophoresis. He received the
1948
Chemistry Nobel Prize for his work on electrophoresis for the separation
of proteins and amino acids. In 1938, American electrical engineer Hendrik Wade Bode (1905-1982) made an
impact in electrochemistry through the Bode plot, which is used extensively in electrochemical impedance analysis of electrochemical systems.
 |
| Pourbaix |
By 1938, Belgian electrochemist
Marcel Pourbaix (1904-1998) had constructed his famous
potential�pH diagrams, also called
�Pourbaix diagrams�. His work underpins
many aspects of corrosion science, electrochemical refining, batteries, electrodeposition,
and electrocatalysis. In 1952 he founded
the Commission of Electrochemistry
of the International Union of Pure
and Applied Chemistry, which in the
following year laid down the rules
that govern the signs of electrode
potentials.
Much of the theory behind cyclic
voltammetry and electrochemical impedance spectroscopy came from the work of the English electrochemist John
Edward Brough Randles (1912-1998). His 1947 work on
the cathode ray polarograph (oscillopolarograph) marked the
beginning of linear sweep voltammetry, the solution to the
peak current that comes through the famous Randles�Sevcik
equation. (Sevcik was a Czech scientist, who along with
Paul Delahay, developed several instruments with triangular
sweeps. As an aside, it must be mentioned that it was Delahay
who in the 1950s introduced chronopotentiometry.) The same
year (1947) Randles published an analysis of an impedance circuit containing diffusion and interfacial electron transfer,
which opened up a method to study fast electrode reactions.
Randles was not given to much publishing, but his papers lent
remarkable insights into the mechanism of electrochemical
processes. The equivalent circuit used in the analysis is known
as the Randles equivalent circuit, but in all fairness must be
termed the Randles�Ershler circuit, for Dolin and Ershler had
published similar results in the Soviet Union in 1940, which,
however, were not easily available to the West due to the
raging Second World War.
Enter British engineer Francis Thomas Bacon. During his
years at C. A. Parsons & Co., Ltd., an electrical company
based in Newcastle-upon-Tyne, he became the first to develop
a practical hydrogen�air fuel cell and suggested its use in submarines. Unlike Grove�s cell, which used an acid electrolyte
and solid electrodes, Bacon�s fuel cell had a less corrosive basic
electrolyte and pressurized porous gas-diffusion electrodes. The first practical application of his technology was to come
years later in Apollo missions, which relied on fuel cells for
in-flight power, heating, and drinking water (a product of the
electrochemical reaction). It is interesting to note that Bacon
was the recipient of the first Grove medal in 1991.
Second half of the twentieth century and later
If energetics of electrochemical systems
dominated the first half of the twentieth century, kinetics of electrochemical reactions began to be recognized as an
important branch of theoretical electrochemistry in the
second half. The credit for connecting electrochemistry and kinetics must go to English physical chemist John Alfred Valentine Butler (1899-1977). He,
along with German surface chemist Max Volmer (1885-1965),
and Hungarian physical chemist Tibor Erdey-Gruz
(1902-1976), laid the seeds of the phenomenological basis
of electrochemical kinetics. The Butler-Volmer equation is a product of their contribution to theoretical electrochemistry.
People in Berlin are familiar with Max-Volmer Institute at the
Technical University of Berlin and the Max-Volmerstrasse. In
1951, Butler teamed up with R. W. Gurney in introducing
the concept of energy levels in electrochemical calculations.
Butler�s contributions to biochemistry are less well known,
especially his work on the kinetics of enzyme action. Electrode
kinetics also gained tremendously through the work of the
German electrochemist Klaus-Jurgen Vetter (1916-1974),
who made path-breaking interpretations of the exchange current density, electrochemical reaction order, and the Flade potential.
 |
| Kolthoff |
In the late 1950s, electroanalytical techniques came of age with the study of the hanging mercury drop electrode
by Polish chemist Wiktor Kemula (1902-1985), whose
seminal work in electroanalytical chemistry led to extensive
investigations in polarography, amalgam electrochemistry,
stripping voltammetry, cyclic voltammetry, and electron transfer. Dutch-American physical and analytical chemist Izaak
Maurits Kolthoff (1927-1962), popularly known as the �father of
analytical chemistry� helped transform analytical chemistry from an art with
empirical recipes to an independent scientific discipline. Among his
contributions to electroanalytical chemistry are conductometric and potentiometric analysis, polarography for environmental trace metal analysis, ion-selective electrodes, electron transfer and precipitation reactions,
and chemistry of nonaqueous media. He nurtured an edifice of analytical chemistry and a galaxy of students, which
included such boldface names as James J. Lingane and
Herbert A. Laitinen. The amalgamation of ideas and concepts
from diverse areas such as energetics, kinetics,
stereochemistry, electrochemistry, pH, and acid-base reactions
that Kolthoff brought into analytical chemistry was of such
import that Lingane once said, �...analytical chemistry has
never been served by a more original mind, nor a more
prolific pen, than Kolthoff�s.�
Parallel developments were also happening in
electrochemical instrumentation, the most significant among
them probably being the invention of the potentiostat by the German engineer-physicist Hans Wenking (b. 1923).
Basing the bulb amplifier, which he designed in 1952, as
the core of his invention, Wenking made his contribution
to electrochemistry through the potentiostat. Until 1957,
his potentiostats were used at the Max Planck Institute
at Gottingen for corrosion studies. Later, Wenking along
with Gerhard Bank, began manufacture of the instrument.
Potentiostats have come a long way in their circuitry, but
the Wenking potentiostat remains a common trade name
and serves electrochemistry in myriad ways: corrosion measurement, controlled electrolysis, and electrochemical passivation.
 |
| Gerischer |
As Germany continued its
contribution toward the growth
of electrochemistry, there emerged
another great name: Heinz Gerischer
(1919-1994). The study of electrochemistry
and photoelectrochemistry
at semiconductor electrodes and the
electrochemistry of excited states owe
their origin to this genius. In 1960 and
1961, he made a detailed mathematical
analysis of redox reactions at metal and semiconductor electrodes, by which he showed that electron transfer
across the electrode-electrolyte interface occurred through
tunneling. With a strong background in chemistry and
physics, he toyed with several concepts, many of which were
only beginning to be unraveled: the double-potential step (or
a.c. modulation) to study very fast electrochemical reactions,
interfacial electrochemistry, single crystal electrodes, and low
energy electron diffraction [which his assistant Gerhard Ertl
(b. 1936) pursued, Ertl received the
2007 Nobel Prize in Chemistry], ultra-high vacuums, and time-resolved
spectroscopy. His work on electrode kinetics covered a lot of
ground in the interpretation of the mechanisms of electrode processes. For example, he introduced the potentiostatic
transient technique for the study of reaction mechanisms.
 |
| Rangarajan |
Scientific research in the just-independent India of
the 1950s was considered more of an oddity of human
endeavor than a profession. But that did not deter Indian
electrochemist Kadarundalige Sitharama Gururaja
Doss (1906-1989) pioneering a.c. effects on adsorption
and electrode kinetics. He also introduced the techniques of tensammetry (independently of Breyer) and the redoxokinetic
effect, now termed faradaic rectification. The impact
of these techniques in adsorption
studies, kinetics of fast reactions, and
electroanalysis of trace metals is well
known. One of his illustrious proteges,
Sarukkai Krishnamachari
Rangarajan (1932-2008) was well
known for his unified approach to
modeling interfacial phenomena at
the macro, molecular, and electronic
levels.
 |
| Conway |
 |
| Bockris |
This period also saw arguably the
finest collaboration between a student
and a teacher in electrochemistry,
featuring Brian Evans Conway
(1927-2005) and John O�Mara
Bockris (b. 1923). They were
instrumental in bringing electrochemistry
from its moribund state
of the Second World War era to
modernity through their monumental
multi-volume Modern Aspects of
Electrochemistry. Incidentally, another
student of Bockris, the Indian
electrochemist Amulya K. N. Reddy
also helped bring electrochemistry
to the fore by compiling Bockris�s
lectures into Modern Electrochemistry,
a two-volume book and arguably a
classic. Bockris and Conway were
the trail-blazers of electrode kinetics
in the western world. Conway was
a �complete� electrochemist in that
he worked on nearly all aspects of
electrochemistry: the electrified
interface, ion solvation, adsorption,
electrode kinetics, oxide film formation,
electrocatalysis, rechargeable batteries,
and electrochemical capacitors.
 |
| Kordesch |
Austrian inventor and electrochemist
Karl V. Kordesch (b. 1922) is a
familiar name to researchers in the
area of batteries and fuel cells. He is
the inventor of the alkaline primary
cell (that has largely replaced the zinc�carbon dry cells in flashlights) and the
key promoter of the RAM (rechargeable
alkaline manganese dioxide) battery
technology. In the 1960s, he became
the first to develop the Apollo Fuel Cell,
an alkaline fuel cell with a circulating electrolyte.
 |
| Marcus |
This was also the time when quantum electrochemistry
was taking roots with path-breaking investigations by
Georgian scientist Revaz Dogonadze (1931-1985). He was
the first to recognize electron transfer processes as quantum
mechanical transitions between two electronic states.
His research group was the first to suggest a quantum mechanical
model for proton transfer in polar solvents, which led
formulation of a quantum mechanical theory of chemical,
electrochemical, and biochemical processes in polar liquids.
But it was Rudolph Marcus (b. 1923)
an American theoretical chemist,
who put quantum electrochemistry on
map. He won the 1992 Nobel Prize in Chemistry for his theory of electron transfer in chemical systems.
Today electrochemistry looks
set to shift gears with parallel
advancements in materials science
and characterization techniques. For
example, there is increasing thrust
toward exploiting nanoscopic materials
and architectures. Expectations in
this direction are high because surface plays a key role in
electrochemical processes. Continual developments in the
synthesis and characterization of materials have also led to
welcome changes in electrochemical research. In closing,
we have made a chronological sequence of noteworthy
developments in electrochemistry although this approach
might rob the interconnections between them. The scientists
who are portrayed as pillars of electrochemistry here have
either made seminal contributions or helped sprout what
were previously only just suggestions. We do hope that our
list is comprehensive; undoubtedly there are inadvertent
omissions. For these we apologize in advance and would
welcome comments.
Acknowledgements
This article was reproduced from The Electrochemical Society Interface (Vol. 17, No. 3, Fall 2008) with permission of The Electrochemical Society, Inc. and the authors.
The authors thank Krishnan Rajeshwar for a critical reading of the
manuscript and many helpful suggestions.
Related articles
Many original journal articles written by the above listed famous electrochemists are available on the WWW at a listing of historical publications in electrochemistry.
Jaroslav Heyrovsky and polarography
Walther Nernst: physicist and chemist
Julius Tafel - his life and science
The Electrochemical Society: The First
Hundred Years, 1902 - 2002
Volta and the "Pile"
Listings of electrochemistry books, review chapters, proceedings volumes, and full text of some historical publications are also available in the Electrochemistry Science and Technology Information Resource (ESTIR). (http://knowledge.electrochem.org/estir/)
Return to:
Top –
Encyclopedia Home Page –
Table of Contents –
Author Index –
Subject Index –
Search –
Dictionary –
ESTIR Home Page –
ECS Home Page
|